Abstract
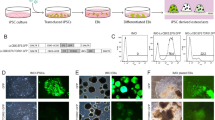
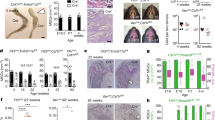
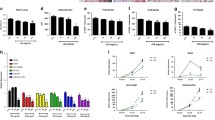
Infantile malignant osteopetrosis (IMO) is a rare, recessive disorder characterized by increased bone mass caused by dysfunctional osteoclasts. The disease is most often caused by mutations in the TCIRG1 gene encoding a subunit of the V-ATPase involved in the osteoclasts capacity to resorb bone. We previously showed that osteoclast function can be restored by lentiviral vector-mediated expression of TCIRG1, but the exact threshold for restoration of resorption as well as the cellular response to vector-mediated TCIRG1 expression is unknown. Here we show that expression of TCIRG1 protein from a bicistronic TCIRG1/GFP lentiviral vector was only observed in mature osteoclasts, and not in their precursors or macrophages, in contrast to GFP expression, which was observed under all conditions. Thus, vector-mediated TCIRG1 expression appears to be post-transcriptionally regulated, preventing overexpression and/or ectopic expression and ensuring protein expression similar to that of wild-type osteoclasts. Codon optimization of TCIRG1 led to increased expression of mRNA but lower levels of protein and functional rescue. When assessing the functional rescue threshold in vitro, addition of 30 % CB CD34+ cells to IMO CD34+ patient cells was sufficient to completely normalize resorptive function after osteoclast differentiation. From both an efficacy and a safety perspective, these findings will clearly be of benefit during further development of gene therapy for osteopetrosis.






Similar content being viewed by others
References
Segovia-Silvestre T, Neutzsky-Wulff AV, Sorensen MG, Christiansen C, Bollerslev J, Karsdal MA, Henriksen K (2009) Advances in osteoclast biology resulting from the study of osteopetrotic mutations. Hum Genet 124:561–577
Tolar J, Teitelbaum SL, Orchard PJ (2004) Osteopetrosis. N Engl J Med 351:2839–2849
Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A (2000) Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9:522–536
Scimeca JC, Franchi A, Trojani C, Parrinello H, Grosgeorge J, Robert C, Jaillon O, Poirier C, Gaudray P, Carle GF (2000) The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26:207–213
Moscatelli I, Thudium CS, Flores C, Schulz A, Askmyr M, Gudmann NS, Andersen NM, Porras O, Karsdal MA, Villa A, Fasth A, Henriksen K, Richter J (2013) Lentiviral gene transfer of TCIRG1 into peripheral blood CD34(+) cells restores osteoclast function in infantile malignant osteopetrosis. Bone 57:1–9
Taranta A, Migliaccio S, Recchia I, Caniglia M, Luciani M, De Rossi G, Dionisi-Vici C, Pinto RM, Francalanci P, Boldrini R, Lanino E, Dini G, Morreale G, Ralston SH, Villa A, Vezzoni P, Del Principe D, Cassiani F, Palumbo G, Teti A (2003) Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am J Pathol 162:57–68
Orchard PJ, Fasth AL, Le Rademacher J, He W, Boelens JJ, Horwitz EM, Al-Seraihy A, Ayas M, Bonfim CM, Boulad F, Lund T, Buchbinder DK, Kapoor N, O’Brien TA, Perez MAD, Veys PA, Eapen M (2015) Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood 126:270–276
Schulz AS, Classen CF, Mihatsch WA, Sigl-Kraetzig M, Wiesneth M, Debatin K-M, Friedrich W, Müller SM (2002) HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood 99:3458–3460
Thudium CS, Jensen VK, Karsdal MA, Henriksen K (2012) Disruption of the V-ATPase functionality as a way to uncouple bone formation and resorption—a novel target for treatment of osteoporosis. Curr Protein Pept Sci [Internet] 13:141–151. http://www.ncbi.nlm.nih.gov/pubmed/22044152
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8(11):917–929
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49(23):4715–4723
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M (2003) From lysosomes to the plasma membrane. Localization of vacuolar type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278:22023–22030
Johansson MK, De Vries TJ, Schoenmaker T, Ehinger M, Brun ACM, Fasth A, Karlsson S, Everts V, Richter J (2007) Hematopoietic stem cell-targeted neonatal gene therapy reverses lethally progressive osteopetrosis in oc/oc mice. Blood 109(12):5178–5185
Sørensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, Karsdal MA (2007) Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Miner Metab 25(1):36–45
Henriksen K, Karsdal MA, Taylor A, Tosh D, Coxon FP (2012) Generation of human osteoclasts from peripheral blood. Methods Mol Biol 816:159–175
Sørensen MG, Henriksen K, Neutzsky-Wulff AV, Dziegiel MH, Karsdal MA (2007) Diphyllin, a novel and naturally potent V-ATPase inhibitor, abrogates acidification of the osteoclastic resorption lacunae and bone resorption. J Bone Miner Res 22:1640–1648
Karsdal MA, Hjorth P, Henriksen K, Kirkegaard T, Nielsen KL, Lou H, Delaissé JM, Foged NT (2003) Transforming growth factor-β controls human osteoclastogenesis through the p38 MAPK and regulation of RANK expression. J Biol Chem 278:44975–44987
Bhargava A, Voronov I, Wang Y, Glogauer M, Kartner N, Manolson MF (2012) Osteopetrosis mutation R444L causes endoplasmic reticulum retention and misprocessing of vacuolar H+-ATPase a3 subunit. J Biol Chem 287:26829–26839
Flores C, De Vries TJ, Moscatelli I, Askmyr M, Schoenmaker T, Langenbach GEJ, Ehinger M, Everts V, Richter J (2010) Nonablative neonatal bone marrow transplantation rapidly reverses severe murine osteopetrosis despite low-level engraftment and lack of selective expansion of the osteoclastic lineage. J Bone Miner Res 25(9):2069–2077
Beranger GE, Momier D, Rochet N, Quincey D, Guigonis J-M, Samson M, Carle GF, Scimeca J-C (2006) RANKL treatment releases the negative regulation of the poly(ADP-ribose) polymerase-1 on Tcirg1 gene expression during osteoclastogenesis. J Bone Miner Res 21:1757–1769
Beranger GE, Momier D, Guigonis J-M, Samson M, Carle GF, Scimeca J-C (2007) Differential binding of poly(ADP-Ribose) polymerase-1 and JunD/Fra2 accounts for RANKL-induced Tcirg1 gene expression during osteoclastogenesis. J Bone Miner Res 22:975–983
Kane PM, Tarsio M, Liu J (1999) Early steps in assembly of the yeast vacuolar H+-ATPase. J Biol Chem [Internet] [cited 2016 May 10], 274:17275–17283. http://www.ncbi.nlm.nih.gov/pubmed/10358087
Glisovic T, Bachorik JL, Yong J, Dreyfuss G (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582(14):1977–1986
Athanasopoulos T, Foster H, Foster K, Dickson G (2011) Codon optimization of the microdystrophin gene for Duchene muscular dystrophy gene therapy. Methods Mol Biol 709:21–37
Suwanmanee T, Hu G, Gui T, Bartholomae CC, Kutschera I, von Kalle C, Schmidt M, Monahan PE, Kafri T (2014) Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIX restore normal hemostasis in Hemophilia B mice. Mol Ther 22:567–574
Mauro VP, Chappell SA (2014) A critical analysis of codon optimization in human therapeutics. Trends Mol Med 20:604–613
Sobacchi C, Pangrazio A, Lopez AG-M, Gomez DP-V, Caldana ME, Susani L, Vezzoni P, Villa A (2014) As little as needed: the extraordinary case of a mild recessive osteopetrosis owing to a novel splicing hypomorphic mutation in the TCIRG1 gene. J Bone Miner Res 29:1646–1650
Palagano E, Blair HC, Pangrazio A, Tourkova I, Strina D, Angius A, Cuccuru G, Oppo M, Uva P, Van Hul W, Boudin E, Superti-Furga A, Faletra F, Nocerino A, Ferrari MC, Grappiolo G, Monari M, Montanelli A, Vezzoni P, Villa A, Sobacchi C (2015) Buried in the middle but guilty: intronic mutations in the TCIRG1 gene cause human autosomal recessive osteopetrosis. J Bone Miner Res 30:1814–1821
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH, Schambach A, Charrier S, Galy A, Thrasher AJ, Bueren J, Baum C (2009) Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 17:1919–1928
Carbonaro DA, Zhang L, Jin X, Montiel-Equihua C, Geiger S, Carmo M, Cooper A, Fairbanks L, Kaufman ML, Sebire NJ, Hollis RP, Blundell MP, Senadheera S, Fu P-Y, Sahaghian A, Chan RY, Wang X, Cornetta K, Thrasher AJ, Kohn DB, Gaspar HB (2014) Preclinical demonstration of lentiviral vector-mediated correction of immunological and metabolic abnormalities in models of adenosine deaminase deficiency. Mol Ther 22:607–622
Acknowledgments
We thank Christopher Baum and Axel Schambach (Hannover Medical School, Germany) for providing us with the lentiviral vector backbone. KH is supported by the Danish Research Foundation (Den Danske Forskningsfond). JR is supported by grants from the Swedish Childhood Cancer Foundation, a Clinical Research Award, from Lund University Hospital, the Foundations of Lund University Hospital. AS is partially supported by grants of the EU (ERARE initiative, project OSTEOPETR). HL is supported by Marie Curie Initial Training Networks (Euroclast, FP7-People-2013-ITN: # 607446). The Lund Stem Cell Center is supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Morten Asser Karsdal owns stock in Nordic Bioscience. Christian Schneider Thudium, Ilana Moscatelli, Henrik Löfvall, Zsuzsanna Kertész, Carmen Montano, Carmen Flores Bjurström, Ansgar Schulz, Johan Richter and Kim Henriksen declare no competing financial interests.
Human and Animal Rights
All procedures involving human subjects were approved by the local Human Investigation Ethics Committee.
Informed Consent
Written informed consent was obtained from all subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thudium, C.S., Moscatelli, I., Löfvall, H. et al. Regulation and Function of Lentiviral Vector-Mediated TCIRG1 Expression in Osteoclasts from Patients with Infantile Malignant Osteopetrosis: Implications for Gene Therapy. Calcif Tissue Int 99, 638–648 (2016). https://doi.org/10.1007/s00223-016-0187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0187-6