Abstract
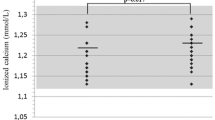
Familial Hypocalciuric Hypercalcaemia (FHH) Type 1 is caused by an inactivating mutation in the calcium-sensing receptor (CASR) gene resulting in elevated plasma calcium levels. We investigated whether FHH is associated with change in bone density and structure. We compared 50 FHH patients with age- and gender-matched population-based controls (mean age 56 years, 69 % females). We assessed areal BMD (aBMD) by DXA-scans and total, cortical, and trabecular volumetric BMD (vBMD) as well as bone geometry by quantitative computed tomography (QCT) and High-Resolution peripheral-QCT (HR-pQCT). Compared with controls, FHH females had a higher total and trabecular hip vBMD and a lower cortical vBMD and hip bone volume. Areal BMD and HRpQCT indices did not differ except an increased trabecular thickness and an increased vBMD at the transition zone between cancellous and cortical bone in of the tibia in FHH. Finite element analyses showed no differences in bone strength. Multiple regression analyses revealed correlations between vBMD and P-Ca2+ levels but not with P-PTH. Overall, bone health does not seem to be impaired in patients with FHH. In FHH females, bone volume is decreased, with a lower trabecular volume but a higher vBMD, whereas cortical vBMD is decreased in the hip. This may be due to either an impaired endosteal resorption or corticalization of trabecular bone. The smaller total bone volume suggests an impaired periosteal accrual, but bone strength is not impaired. The findings of more pronounced changes in females may suggest an interaction between sex hormones and the activity of the CaSR on bone.
Similar content being viewed by others
References
Ward BK, Magno AL, Walsh JP, Ratajczak T (2012) The role of the calcium-sensing receptor in human disease. Clin Biochem 45(12):943–953
Nesbit MA, Hannan FM, Howles SA, Reed AA, Cranston T, Thakker CE, Gregory L, Rimmer AJ, Rust N, Graham U, Morrison PJ, Hunter SJ, Whyte MP, McVean G, Buck D, Thakker RV (2013) Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet 45:93–97
Nesbit MA, Hannan FM, Howles SA, Babinsky VN, Head RA, Cranston T, Rust N, Hobbs MR, Heath H 3rd, Thakker RV (2013) Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. N Engl J Med 368:2476–2478
Christensen SE, Nissen PH, Vestergaard P, Mosekilde L (2011) Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabete Obes 18:359–370
Jakobsen NF, Rolighed L, Nissen PH, Mosekilde L, Rejnmark L (2013) Muscle function and quality of life is not impaired in familial hypocalciuric hypercalcemia (FHH): a cross-sectional study on physiological effects of inactivating variants in the calcium sensing receptor gene (CaSR). Eur J Endocrinol 169(3):349–357
Chang W, Tu C, Chen TH, Bikle D, Shoback D (2008) The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1:ra1
Law WM Jr, Wahner HW, Heath H 3rd (1984) Bone mineral density and skeletal fractures in familial benign hypercalcemia (hypocalciuric hypercalcemia). Mayo Clin Proc 59:811–815
Kristiansen JH, Rodbro P, Christiansen C, Johansen J, Jensen JT (1987) Familial hypocalciuric hypercalcaemia. III: bone mineral metabolism. Clin Endocrinol (Oxf) 26:713–716
Abugassa S, Nordenstrom J, Jarhult J (1992) Bone mineral density in patients with familial hypocalciuric hypercalcaemia (FHH). Eur J Surg 158:397–402
Menko FH, Bijvoet OL, Fronen JL, Sandler LM, Adami S, O’Riordan JL, Schopman W, Heynen G (1983) Familial benign hypercalcaemia. Study of a large family. Q J Med 52:120–124
Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Rejnmark L, Brixen K, Mosekilde L (2009) Skeletal consequences of familial hypocalciuric hypercalcaemia versus primary hyperparathyroidism. Clin Endocrinol (Oxf) 71:798–807
Theman TA, Collins MT, Dempster DW, Zhou H, Reynolds JC, Brahim JS, Roschger P, Klaushofer K, Winer KK (2009) PTH(1-34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res 24:964–973
Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HA, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FM, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JP, Spector TD, Genetic Factors for Osteoporosis Consortium (2009) Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151:528–537
Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Brixen K, Mosekilde L (2008) Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin Endocrinol (Oxf) 69:713–720
Isaksen T, Nielsen CS, Christensen SE, Nissen PH, Heickendorff L, Mosekilde L (2011) Forearm bone mineral density in familial hypocalciuric hypercalcemia and primary hyperparathyroidism: a comparative study. Calcif Tissue Int 89:285–294
Hansen S, Beck Jensen JE, Rasmussen L, Hauge EM, Brixen K (2010) Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: a case-control study using HR-pQCT. J Bone Miner Res 25:1941–1947
Harding B, Curley AJ, Hannan FM, Christie PT, Bowl MR, Turner JJ, Barber M, Gillham-Nasenya I, Hampson G, Spector TD, Thakker RV (2006) Functional characterization of calcium sensing receptor polymorphisms and absence of association with indices of calcium homeostasis and bone mineral density. Clin Endocrinol (Oxf) 65:598–605
Nissen PH, Christensen SE, Heickendorff L, Brixen K, Mosekilde L (2007) Molecular genetic analysis of the calcium sensing receptor gene in patients clinically suspected to have familial hypocalciuric hypercalcemia: phenotypic variation and mutation spectrum in a Danish population. J Clin Endocrinol Metab 92:4373–4379
Hermann AP, Thomsen J, Vestergaard P, Mosekilde L, Charles P (1999) Assessment of calcium intake. A quick method comparerd to a 7 days food diary. Calcif Tissue Int 64(suppl 1):S82
Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L (2011) Determinants of plasma PTH and their implication for defining a reference interval. Clin Endocrinol (Oxf) 74:37–43
Hojskov CS, Heickendorff L, Moller HJ (2010) High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25(OH) vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta 411:114–116
Nissen PH, Christensen SE, Ladefoged SA, Brixen K, Heickendorff L, Mosekilde L (2012) Identification of rare and frequent variants of the CASR gene by high-resolution melting. Clin Chim Acta 413:605–611
den Dunnen JT, Antonarakis SE (2001) Nomenclature for the description of human sequence variations. Hum Genet 109:121–124
Abrahamsen B, Gram J, Hansen TB, Beck-Nielsen H (1995) Cross calibration of QDR-2000 and QDR-1000 dual-energy X-ray densitometers for bone mineral and soft-tissue measurements. Bone 16:385–390
Sikjaer T, Rejnmark L, Thomsen JS, Tietze A, Bruel A, Andersen G, Mosekilde L (2012) Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1-84): a randomized controlled study. J Bone Miner Res 27:781–788
Sode M, Burghardt AJ, Pialat JB, Link TM, Majumdar S (2011) Quantitative characterization of subject motion in HR-pQCT images of the distal radius and tibia. Bone 48:1291–1297
Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S (2012) Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone 50:111–118
Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P (1997) Ridge number density: a new parameter for in vivo bone structure analysis. Bone 21:541–546
Laib A, Ruegsegger P (1999) Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24:35–39
Hansen S, Hauge EM, Rasmussen L, Jensen JE, Brixen K (2012) Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism: a one-year prospective controlled study using high-resolution peripheral quantitative computed tomography. J Bone Miner Res 27:1150–1158
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41:1–505
Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK (2010) Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res 25:882–890
Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S (2010) Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res 25:983–989
Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P (2002) Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 30:842–848
Seeman E (2003) Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med 349:320–323
Lotinun S, Evans GL, Bronk JT, Bolander ME, Wronski TJ, Ritman EL, Turner RT (2004) Continuous parathyroid hormone induces cortical porosity in the rat: effects on bone turnover and mechanical properties. J Bone Miner Res 19:1165–1171
Marie PJ (2010) The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone 46:571–576
Wu S, Palese T, Mishra OP, Delivoria-Papadopoulos M, De Luca F (2004) Effects of Ca2+ sensing receptor activation in the growth plate. FASEB J 18:143–145
Brown EM (2013) Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab 27:333–334
Broome JT, Solorzano CC (2011) Lithium use and primary hyperparathyroidism. Endocr Pract 17(Suppl 1):31–35
Brown EM (1981) Lithium induces abnormal calcium-regulated PTH release in dispersed bovine parathyroid cells. J Clin Endocrinol Metab 52:1046–1048
Zamani A, Omrani GR, Nasab MM (2009) Lithium’s effect on bone mineral density. Bone 44:331–334
Vestergaard P, Rejnmark L, Mosekilde L (2005) Reduced relative risk of fractures among users of lithium. Calcif Tissue Int 77:1–8
Allagui MS, Hfaiedh N, Croute F, Guermazi F, Vincent C, Soleilhavoup JP, El Feki A (2005) Side effects of low serum lithium concentrations on renal, thyroid, and sexual functions in male and female rats. C R Biol 328:900–911
Hobson SA, McNeil SE, Lee F, Rodland KD (2000) Signal transduction mechanisms linking increased extracellular calcium to proliferation in ovarian surface epithelial cells. Exp Cell Res 258:1–11
Journe F, Dumon JC, Kheddoumi N, Fox J, Laios I, Leclercq G, Body JJ (2004) Extracellular calcium downregulates estrogen receptor alpha and increases its transcriptional activity through calcium-sensing receptor in breast cancer cells. Bone 35:479–488
Leclercq G (2012) Calcium-induced activation of estrogen receptor alpha—new insight. Steroids 77:924–927
Ba J, Brown D, Friedman PA (2003) Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol 285:F1233–F1243
Acknowledgements
This work was supported by a Grant (#10-094047) from The Danish Council for independent Research in Medical Sciences (FSS). The study was conducted at The Osteoporosis Clinic, Aarhus University Hospital, and we hereby thank all the staff for support during the investigations.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakobsen, N.F.B., Rolighed, L., Moser, E. et al. Increased Trabecular Volumetric Bone Mass Density in Familial Hypocalciuric Hypercalcemia (FHH) Type 1: A Cross-Sectional Study. Calcif Tissue Int 95, 141–152 (2014). https://doi.org/10.1007/s00223-014-9877-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9877-0