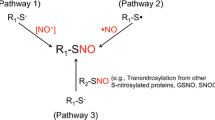
Abstract
Posttranslational modifications of cysteine sulfhydryl (–SH) moieties, e.g., S-nitrosylation, S-glutathionylation, or S-sulfuration, play an important role in cellular response to oxidative stress. Reversible cysteine modifications alter protein function and can play a critical role in redox signal transduction. Perturbation of sulfhydryl homeostasis is a hallmark of many diseases, including neurodegenerative disorders. Besides direct oxidative stress within the neurons, inflammation of the central nervous system as well as the periphery is implicated also in the development and progression of neurodegeneration. Therefore, perturbation of redox regulation of key inflammatory mediators is an important component of neurodegenerative diseases. Many proteins involved in inflammation have been shown to undergo S-nitrosylation (–SNO) and/or S-glutathionylation (–SSG) with functional consequences. The mechanistic and functional relationships between these two modifications have yet to be thoroughly investigated. While protein–SNO intermediates in some cases may signal independently of protein–SSG intermediates, the relatively unstable nature of protein–SNO derivatives in the presence of GSH suggests that protein–SNO formation in many cases may serve as a precursor for protein–SSG modifications. In this review, we describe the cysteine modifications of specific inflammation-mediating proteins and their relationship to inflammatory responses such as cytokine and chemokine production. In particular, we consider evidence for sequential protein–SNO → protein–SSG modifications of these proteins. We conclude that cysteine modifications of critical regulatory proteins are likely to play a central role in the onset and progression of neuroinflammatory diseases and thus should be studied thoroughly in this context.




Similar content being viewed by others
Abbreviations
- ACT:
-
α1-Antichymotrypsin
- AD:
-
Alzheimer’s disease
- Akt/PKB:
-
Protein kinase B
- ALS:
-
Amyotrophic lateral sclerosis
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid-beta
- BioGEE:
-
Biotinylated glutathione ethyl ester
- Biotin-HPDP:
-
N-[(6-biotinamido)hexyl]-3′-(2′-pyridyldithio) propionamide
- BMDM:
-
Bone marrow derived macrophages
- CD4:
-
Cluster of differentiation 4
- CNS:
-
Central nervous system
- COX2:
-
Cyclooxygenase 2
- CSE:
-
Cystathionine γ-lyase
- CSF:
-
Cerebrospinal fluid
- CXCR3/4:
-
Chemokine receptor 3/4
- Cys-SNO:
-
S-nitrosocysteine
- DAMPs:
-
Damage-associated molecular patterns
- DTT:
-
Dithiothreitol
- EAE:
-
Experimental autoimmune encephalomyelitis
- eNOS:
-
Endothelial nitric oxide synthase
- ERK1/2:
-
Extracellular-signal-regulated kinase 1/2
- GCL:
-
Glutamate cysteine ligase
- GGCS:
-
Gamma-glutamylcysteine synthetase
- Grx:
-
Glutaredoxin
- Grx1:
-
Glutaredoxin-1
- GS· :
-
Glutathione radical
- GSH:
-
Glutathione
- GSNO:
-
S-Nitrosoglutathione
- GSSG:
-
Glutathione disulfide
- GST:
-
Glutathione-S-transferase
- GSTπ:
-
Glutathione S-transferase pi
- H2S:
-
Hydrogen sulfide
- HD:
-
Huntington’s disease
- HMGB1:
-
High-mobility group protein B1
- HO-1:
-
Heme oxygenase 1
- IAM:
-
Iodoacetamide
- Iba:
-
Ionized calcium-binding adaptor molecule 1
- ICE:
-
Interleukin-1 converting enzyme
- IKKα:
-
Inhibitor of nuclear factor kappa B kinase subunit alpha
- IKKβ:
-
Inhibitor of nuclear factor kappa B kinase subunit beta
- IL-1R:
-
Interleukin 1 receptor
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- INFγ:
-
Interferon gamma
- iNOS:
-
Inducible nitric oxide synthase
- IRAK:
-
Interleukin-1 receptor-associated kinase
- IRF3:
-
Interferon regulatory factor 3
- IκBα:
-
Inhibitory kappa B alpha
- IκBα:
-
Nuclear factor of kappa-light polypeptide gene enhancer in B cells inhibitor, alpha
- JNK:
-
c-Jun N-terminal kinase
- LPS:
-
Lipopolysaccharide
- LRRK2:
-
Leucine-rich repeat kinase 2
- LTβR:
-
Lymphotoxin-beta receptor
- MMTS:
-
S-Methyl methanethiosulfonate
- MND:
-
Motor neuron disease
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS:
-
Multiple sclerosis
- MyD88:
-
Myeloid differentiation primary response gene (88)
- NADP+/H:
-
Nicotinamide adenine dinucleotide phosphate (oxidized/reduced)
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NFκB2/p100:
-
Nuclear factor NF-kappa B p100 subunit
- NLRP3:
-
NACHT, LRR and PYD domains-containing protein 3
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- NSAID:
-
Non-steroid anti-inflammatory drug
- PAMPs:
-
Pathogen-associated molecular patterns
- PD:
-
Parkinson’s disease
- PKB:
-
Protein kinase B
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- Pro-SH:
-
Reduced protein thiol
- Pro-SNO:
-
S-nitrosylated protein
- Pro-SO2H:
-
Protein sulfinic acid
- Pro-SO3H:
-
Protein sulfonic acid
- Pro-SOH:
-
Protein sulfenic acid
- Pro-SSG:
-
Glutathionylated protein
- Pro-SSH:
-
Sulfhydrated protein
- PS1:
-
Presenilin-1
- PTEN:
-
Phosphatase and tensin analog deleted from chromosome 10
- Rac1:
-
Ras-related C3 botulinum toxin substrate 1
- RING:
-
Really interesting new gene
- ROS:
-
Reactive oxygen species
- RTK:
-
Receptor tyrosine kinase
- S100:
-
Soluble in 100 % ammonium sulfate at neutral pH
- S100A8:
-
S100 calcium-binding protein A8
- S100A9:
-
S100 calcium-binding protein A9
- SNAP:
-
S-nitroso-N-acetylpenicillamine
- SOD:
-
Superoxide dismutase
- solTNF-α:
-
Soluble TNF-α
- STAT3:
-
Signal transducer and activator of transcription 3
- TAB:
-
TAK1-binding protein
- TAK1:
-
TGF (transforming growth factor) beta-activated kinase 1
- TLR:
-
Toll-like receptor
- tmTNF-α:
-
Transmembrane TNF-α
- TNF-α:
-
Tumor necrosis factor alpha
- TNFR:
-
Tumor necrosis factor receptor
- TR:
-
Thioredoxin reductase
- TRAF6:
-
Tumor necrosis factor receptor-associated factor 6
- Trx:
-
Thioredoxin
References
Adachi T et al (2004) S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10(11):1200–1207
Aesif SW, Janssen-Heininger YMW, Reynaert NL (2010) Protocols for the detection of s-glutathionylated and s-nitrosylated proteins in situ, 1st edn. Elsevier, Amsterdam
Aesif SW et al (2011) Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol 44(4):491–499
Anand A, Thakur K, Gupta PK (2013) ALS and oxidative stress: the neurovascular scenario. Oxid Med Cell Longev 2013:635831. doi:10.1155/2013/635831
Anneser J et al (2004) Glial proliferation and metabotropic glutamate receptor expression in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 63(8):831–840
Aoyama K, Nakaki T (2013) Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci 14(10):21021–21044
Appel SH et al (2011) The microglial-motoneuron dialogue in ALS. Acta myologica: myopathies and cardiomyopathies: official journal of the Mediterranean Society of Myology/edited by the Gaetano Conte Academy for the study of striated muscle diseases 30(1):4–8
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Bansal G et al (2013) IL-22 activates oxidant signaling in pulmonary vascular smooth muscle cells. Cell Signal 25(12):2727–2733
Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25(6):280–288
Bradford J et al (2010) Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem 285(14):10653–10661
Breidert T et al (2002) Protective action of the peroxisome proliferator-activated receptor-γ agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem 82(3):615–624. doi:10.1046/j.1471-4159.2002.00990.x
Brooks BR (2009) Managing amyotrophic lateral sclerosis: slowing disease progression and improving patient quality of life. Ann Neurol 65(Suppl 1):S17–S23
Browne SE, Beal MF (2006) Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal 8(11–12):2061–2073
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19(8):823–835
Butturini E et al (2013) Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic Biol Med 65:1322–1330
Butturini E et al (2014) S-glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem Biol 9(8):1885–1893
Carvalho AN et al (2014) Glutathione in multiple sclerosis: more than just an antioxidant? Mult Scler (Houndmills, Basingstoke, England) 20(11):1425–1431
Casoli T et al (2010) Peripheral inflammatory biomarkers of Alzheimer’s disease: the role of platelets. Biogerontology 11(5):627–633
Chantzoura E et al (2010) Glutaredoxin-1 regulates TRAF6 activation and the IL-1 receptor/TLR4 signalling. Biochem Biophys Res Commun 403(3–4):335–339
Chung S et al (2010) Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of IκB kinases in mice: impact on histone acetylation. Am J Physiol Lung Cell Mol Physiol 299(2):L192–L203
Chung J-Y et al (2013) Elevated TRAF2/6 expression in Parkinson’s disease is caused by the loss of Parkin E3 ligase activity. Lab Investig 93(6):663–676
Collins LM et al (2012) Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 62(7):2154–2168
Conrad M et al (2013) Glutathione and thioredoxin dependent systems in neurodegenerative disease : What can be learned from reverse genetics in mice. Neurochem Int 62:738–749
Cruz CM et al (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282(5):2871–2879
Cudkowicz ME et al (2006) Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol 60(1):22–31
Czirr E, Wyss-Coray T (2012) The immunology of neurodegeneration. J Clin Invest 122(4):1156–1163
D’Ambrosi N et al (2014) Rac1 at the crossroad of actin dynamics and neuroinflammation in Amyotrophic Lateral Sclerosis. Front Cell Neurosci 8:279
Dalle-Donne I et al (2002) Reversible S-glutathionylation of Cys374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med 34(1):23–32
Deleidi M, Gasser T (2013) The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci CMLS 70(22):4259–4273
Derudder E et al (2003) RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. J Biol Chem 278(26):23278–23284
Diamant G, Dikstein R (2013) Transcriptional control by NF-κB: elongation in focus. Biochim et biophys Acta 1829:937–945
Díaz-amarilla P et al (2011) Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 108(44):18126–18131
Dimmeler BS et al (1997) suppression of apoptosis by nitric oxide via inhibition of interleukin-1b–converting enzyme (ICE)-like and cysteine protease protein (CPP)-32—like proteases. J Exp Med 185(4):601–607
Doens D, Fernández PL (2014) Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J Neuroinflammation 11:48
Dzamko N et al (2012) The IkappaB kinase family phosphorylates the Parkinson’s disease kinase LRRK2 at Ser935 and Ser910 during toll-like receptor signaling. PloS One 7(6):e39132
Edgeworth J et al (1991) Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem 266(12):7706–7713
Elliott JM et al (2009) Crystal structure of procaspase-1 zymogen domain reveals insight into inflammatory caspase autoactivation. J Biol Chem 284(10):6546–6553
Estelle R, Parain K, Chantal J (2002) Role of TNF-α receptors in mice intoxicated with the parkinsonian toxin MPTP. Exp Neurol 192:183–192
Evans MC et al (2013) Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci 53:34–41
Ferger B et al (2004) Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem 89(4):822–833
Fiaschi T et al (2006) Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem 281(32):22983–22991
Findlay VJ et al (2006) A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res 66(13):6800–6806
Finn NA, Kemp ML (2012) Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Mol Biosys 8(2):650–662
Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15(9):391–404
Gallogly MM, Mieyal JJ (2007) Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7(4):381–391
Gallogly MM, Starke DW, Mieyal JJ (2009) Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxidants & redox signaling 11(5):1059–1081
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012:428010–428021
Gao F et al (2013a) Rotenone directly induces BV2 cell activation via the p38 MAPK pathway. PloS One 8(8):e72046
Gao X-H et al (2013b) Aging-dependent changes in rat heart mitochondrial glutaredoxins—implications for redox regulation. Redox Biol 1(1):586–598
Garcia-Garcia A et al (2012) Thiol-redox signaling, dopaminergic cell death, and Parkinson’s disease. Antioxid Redox Signal 17(12):1764–1784
Geetha T et al (2012) TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem Int 61(8):1289–1293
Gilgun-Sherki Y, Melamed E, Offen D (2006) Anti-Inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr Pharm Des 12(27):3509–3519
Goyette J, Geczy CL (2011) Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 41(4):821–842
Greiner R et al (2013) Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19(15):1749–1765
Griffin WST (2008) Perispinal etanercept: potential as an Alzheimer therapeutic. J Neuroinflammation 5:3
Grilli M et al (1995) Identification and characterization of a B/Rel binding site in the regulatory region of the amyloid precursor protein gene. J Neurosci 270(45):26774–26777. doi:10.1074/jbc.270.45.26774
Group AR et al (2008) Cognitive function over time in the Alzheimer’s disease anti-inflammatory prevention trial (ADAPT). Arch Neurol 65(7):896–905
Grumbach IM et al (2005) A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol 39(4):595–603
Gupta V, Carroll KS (2014) Sulfenic acid chemistry, detection and cellular lifetime. Biochim Biophys Acta 2:847–875
Guzmán-Martínez L, Farías GA, Maccioni RB (2012) Emerging noninvasive biomarkers for early detection of Alzheimer’s disease. Arch Med Res 43(8):663–666
Gveric D et al (1998) Transcription factor NF-kappaB and inhibitor I kappaBalpha are localized in macrophages in active multiple sclerosis lesions. J Neuropathol Exp Neurol 57(2):168–178
Hall CN, Garthwaite J (2009) What is the real physiological NO concentration in vivo? Nitric Oxide 21:92–103
Hall J et al (2014) Biomarkers of vascular risk, systemic inflammation and microvascular pathology and neuropsychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis 35(2):363–371
Halle A et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9(8):857–865
Halliwell B (2006) Oxidative stress and neurodegeneration: Where are we now? J Neurochem 97(6):1634–1658
Halloran M, Parakh S, Atkin JD (2013) The role of s-nitrosylation and s-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int J Cell Biol 2013:797914. doi:10.1155/2013/797914
Harms AS et al (2011) Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol Ther 19(1):46–52
Harraz MM et al (2008) SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Investig 118(2):659–670
Heneka MT et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678
Hernandez-Cuellar E et al (2012) Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J Immunol (Baltimore, Md.: 1950) 189(11):5113–5117
Hess DT et al (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6(2):150–166
Hilliard B et al (1999) Experimental autoimmune encephalomyelitis in NF-kappa B-deficient mice: roles of NF-kappa B in the activation and differentiation of autoreactive T cells. J Immunol 163(5):2937–2943
Ho Y et al (2008) Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med 43(9):1299–1312
Hollingworth P et al (2011) Alzheimer’s disease genetics: current knowledge and future challenges. Int J Geriatr Psychiatry 26(8):793–802
Huang Y et al (2005) NF-kappaB precursor, p105, and NF-kappaB inhibitor, IkappaBgamma, are both elevated in Alzheimer disease brain. Neurosci Lett 373(2):115–118
Huh SH et al (2011) Ethyl pyruvate rescues nigrostriatal dopaminergic neurons by regulating glial activation in a mouse model of Parkinson’s disease. J Immunol (Baltimore, Md.: 1950) 187(2):960–969
Hull J et al (2015) Regional Increase in the expression of the BCAT proteins in Alzheimer’s disease brain: implications in glutamate toxicity. J Alzheimers Dis. doi:10.3233/JAD-142970
Ii M et al (1996) beta-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res 720(1–2):93–100
Imai Y, Kohsaka S (2002) Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia 40(2):164–174
Into T et al (2008) Regulation of MyD88-dependent signaling events by S nitrosylation retards toll-like receptor signal transduction and initiation of acute-phase immune responses. Mol Cell Biol 28(4):1338–1347
Ishibashi K et al (2006) Absence of synaptophysin near cortical neurons containing oligomer Abeta in Alzheimer’s disease brain. J Neurosci Res 636(April):632–636
Jaffrey SR et al (2001) Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3(February):193–197
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4(10):1399–1440
Johri A, Beal MF (2012) Antioxidants in Huntington’s disease. Biochim et Biophys Acta 5:664–674
Jung C et al (2001) Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett 304(3):157–160
Kanwar J (2005) Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease. Curr Med Chem 12(25):2947–2962
Kashfi K (2012) Nitric oxide–releasing hybrid drugs target cellular processes through S-nitrosylation. For Immunopathol Dis Therap 3(2):97–108
Katsuyama K, Hirata Y (2001) A pyrrolidinone derivative inhibits cytokine-induced iNOS expression and NF-KB activation by preventing phosphorylation and degradation of IκB-α. J Biochem 129(4):585–591
Katsuyama K et al (1998) NO inhibits cytokine-induced iNOS expression and NF-kB activation by interfering with phosphorylation and degradation of IkB-a. Arterioscler Thromb Vasc Biol 18(11):1796–1802. doi:10.1161/01.ATV.18.11.1796
Kawamata J, Shimohama S (2011) Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis 24(Suppl 2):95–109
Kelleher ZT et al (2007) NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem 282(42):30667–30672
Kelleher ZT et al (2014) Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor κB (NF-κB) activation. J Biol Chem 289(5):3066–3072
Kenchappa RS et al (2004) Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J 18(10):1102–1104
Khoshnan A, Patterson PH (2011) The role of IκB kinase complex in the neurobiology of Huntington’s disease. Neurobiol Dis 43(2):305–311
Kil IS, Kim SY, Park J-W (2008) Glutathionylation regulates IkappaB. Biochem Biophys Res Commun 373(1):169–173
Kim Y et al (1998) Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1(IL-1β-converting enzyme). J Immunol 161:4122–4128
Kim N-H et al (2007) Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 122(4):607–614
Kim J, Won JS, Singh AK, Sharma AK, Singh I (2014) STAT3 regulation by S-nitrosylation: implication for inflammatory disease. Antioxid Redox Signal 20(16):2514–2527
Kordula T et al (2000) Mechanism of interleukin-1- and tumor necrosis factor alpha-dependent regulation of the alpha 1-antichymotrypsin gene in human astrocytes. J Neurosci 20(20):7510–7516
Kwak Y-D et al (2010) NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener 5:49
Kwan W et al (2012) Mutant huntingtin impairs immune cell migration in Huntington disease. J Clin Investig 122(12):4737–4747
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13(6):397–411
Leng A et al (2005) Tumor necrosis factor-alpha receptor ablation in a chronic MPTP mouse model of Parkinson’s disease. Neurosc Lett 375(2):107–111
Lian K-C et al (2010) Dual mechanisms of NF-kappaB inhibition in carnosol-treated endothelial cells. Toxicol Appl Pharmacol 245(1):21–35
Liao B-C et al (2010) The glutaredoxin/glutathione system modulates NF-kappaB activity by glutathionylation of p65 in cinnamaldehyde-treated endothelial cells. Toxicological sciences: an official journal of the Society of Toxicology 116(1):151–163
Lim SY et al (2010) S-glutathionylation regulates inflammatory activities of S100A9. J Biol Chem 285(19):14377–14388
Lim SY et al (2013) S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol 181:5627–5636
Lin Y-C et al (2012) The glutathionylation of p65 modulates NF-κB activity in 15-deoxy-Δ12,14-prostaglandin J2-treated endothelial cells. Free Rad Biol Med 52(9):1844–1853
Lo Conte M, Carroll KS (2013) The chemistry of thiol oxidation and detection. In: Jakob U, Reichmann D (eds) Oxidative stress and redox regulation. Springer, Berlin, pp 1–42
Lu C et al (2013) S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: a common paradigm for gasotransmitter signaling by H2S and NO. Methods 62(2):177–181
Martínez-Ruiz A, Lamas S (2007) Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res 75(2):220–228
Mattson MP, Camandola S (2001) NF-κ B in neuronal plasticity and neurodegenerative disorders. J Clin Investig 107(3):247–254
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45
McCoy MK et al (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci 26(37):9365–9375
McGeer PL, Rogers J, McGeer EG (2006) Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis 9(3 Suppl):271–276
Meissner F, Molawi K, Zychlinsky A (2008) Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol 9(8):866–872
Mieyal JJ, Chock PB (2012) Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on S-glutathionylation. Antioxid Redox Signal 16(6):471–475
Mieyal JJ et al (2008) Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10(11):1941–1988
Mitchell RM et al (2009) A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology 72(1):14–19
Mogi M et al (1994) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165(1–2):208–210
Monson NL et al (2014) Elevated CNS inflammation in patients with preclinical Alzheimer’s disease. J Cereb Blood Flow Metab 34(1):30–33
Mowbray M et al (2008) Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. J Investig Dermatol 128(2):352–360
Murata H et al (2003) Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem 278(50):50226–50233
Murphy MP (2012) Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal 16(6):476–495
Mustafa AK et al (2009) H2S signals through protein S-sulfhydration. Sci Signal 2(96):72
Nikitovic D, Holmgren A (1996) S-Nitrosoglutathione is cleaved by the thioredoxin system with liberation of glutathione and redox regulating nitric oxide. J Biol Chem 271(32):19180–19185. doi:10.1074/jbc.271.32.19180
Nolin JD et al (2014) The glutaredoxin/S-glutathionylation axis regulates interleukin-17A-induced proinflammatory responses in lung epithelial cells in association with S-glutathionylation of nuclear factor κ B family proteins. Free Rad Biol Med 73:143–153
Numajiri N et al (2011) On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN). Proc Natl Acad Sci U S A 108(25):10349–10354
Oeckinghaus A, Hayden MS, Ghosh S (2011) Crosstalk in NF-κB signaling pathways. Nat Immunol 12(8):695–708
Olmos G, Lladó J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat Inflamm 2014:861231. doi:10.1155/2014/861231
Ortiz GG et al (2013) Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clin Dev Immunol 2013:708659
Ozawa K et al (2013) S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci Rep 3:2202
Pantano C et al (2006) Redox-sensitive kinases of the nuclear factor-kB signaling pathway. Antioxid Redox Signal 8(9,10):1791–1806
Park JW, Mieyal JJ, Rhee SG, Chock PB (2009) Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem 284(35):23364–23374
Pei D-S, Sun Y-F, Song Y-J (2009) S-nitrosylation of PTEN invovled in ischemic brain injury in rat hippocampal CA1 region. Neurochem Res 34(8):1507–1512
Peng H, Libby P, Liao J (1995) Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem 270(23):14214–14219
Pennisi G et al (2011) Redox regulation of cellular stress response in multiple sclerosis. Biochem Pharmacol 82(10):1490–1499
Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7(2):161–167
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10(3):253–263
Pineda-Molina E et al (2001) Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40(47):14134–14142
Poole LB, Nelson KJ (2009) Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol 12(1):18–24
Prinarakis E et al (2008) S-glutathionylation of IRF3 regulates IRF3-CBP interaction and activation of the IFN beta pathway. EMBO J 27(6):865–875
Qanungo S et al (2007) Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem 282(25):18427–18436
Qanungo S et al (2014) N-acetyl-l-cysteine sensitizes pancreatic cancers to gemcitabine by targeting the NFκB pathway. Biomed Pharmacother 68(7):855–864
Qian J, Fulton DJR (2012) Exogenous, but not endogenous nitric oxide inhibits adhesion molecule expression in human endothelial cells. Front Physiol 3:3
Raftery MJ et al (2001) Novel intra- and inter-molecular sulfinamide bonds in S100A8 produced by hypochlorite oxidation. J Biol Chem 276(36):33393–33401
Reinhart PH et al (2011) Identification of anti-inflammatory targets for Huntington’s disease using a brain slice-based screening assay. Neurobiol Dis 43(1):248–256
Reisz JA et al (2013) Thiol-blocking electrophiles interfere with labeling and detection of protein sulfenic acids. FEBS J 280(23):6150–6161
Rees K et al (2011) Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: evidence from observational studies (review). Cochrane Database Syst Rev (11):CD008454. doi:10.1002/14651858.CD008454
Reynaert NL et al (2004) Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 101(24):8945–8950
Reynaert NL et al (2006) Dynamic redox control of NF- kappa B through of inhibitory kappaB kinase beta. PNAS 103(35):13086–13091
Rogers J et al (2007) Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? Int Rev Neurobiol 82(07):235–246
Rojas J et al (2010) Interferon beta for primary progressive multiple sclerosis (review). Cochrane Database Syst Rev (1):CD006643. doi:10.1002/14651858.CD006643
Romero J, Bizzozero O (2011) Intracellular glutathione mediates the denitrosylation of protein nitrosothiols in the rat spinal cord. J Neurosci Res 87(3):701–709
Roy A et al (2012) Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PloS One 7(6):e38113
Sabens Liedhegner EA, Gao X-H, Mieyal JJ (2012) Mechanisms of altered redox regulation in neurodegenerative diseases—focus on S-glutathionylation. Antioxid Redox Signal 16(6):543–566
Sahin E et al (2014) Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol (Baltimore, Md.: 1950) 193(4):1717–1727
Saijo K et al (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59
Sakai J et al (2012) Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37:1037–1049
Salter MW, Beggs S (2014) Sublime microglia: expanding roles for the guardians of the CNS. Cell 158(1):15–24
Sapp E et al (2001) Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol 60(2):161–172
Saresella M et al (2014) A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J Alzheimers Dis 38(2):403–413
Schabbauer G et al (2010) Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol (Baltimore, Md.: 1950) 185(1):468–476
Schwartz M, Shechter R (2010) Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol 6(7):405–410
Sen N et al (2013) Hydrogen sulfide-linked sulfhydration of NF-kB mediates its anti-apoptotic actions. Mol Cell Biol 45(1):13–24
Senftleben U et al (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science (New York, N.Y.) 293(5534):1495–1499
Sengupta R, Holmgren A (2013) Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid Redox Signal 18(3):259–269
Sengupta R et al (2007) Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry 46:8472–8483
Shelton MD, Mieyal JJ (2012) Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cell 25(3):332–346
Shelton MD et al (2009) Glutaredoxin regulates autocrine and paracrine proinflammatory responses in retinal glial (muller) cells. J Biol Chem 284(8):4760–4766
Slomiany BL, Slomiany A (2011) Helicobacter pylori induces disturbances in gastric mucosal Akt activation through inducible nitric oxide synthase-dependent S-nitrosylation: effect of ghrelin. ISRN Gastroenterol 2011:308727. doi:10.5402/2011/308727
Sofic E et al (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett 142(2):128–130
Soulet D, Cicchetti F (2011) The role of immunity in Huntington’s disease. Mol Psychiatry 16(9):889–902
Starke DW, Chock PB, Mieyal JJ (2003) Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem 278(17):14607–14613
Sullivan DM et al (2000) Identification of oxidant-sensitive proteins: TNF-α induces protein glutathiolation. Biochemistry 39(36):11121–11128. doi:10.1021/bi0007674
Sun S-C (2013) Noncanonical NF-kB pathway. Immunol Rev 246(1):125–140
Sutherland GT et al (2013) Oxidative stress in Alzheimer’s disease: primary villain or physiological by-product? Redox Rep Commun Free Rad Res 18(4):134–141
Suzumura A (2013) Neuron-microglia interaction in neuroinflammation microglia are double-edged sword. Curr Protein Pept Sci 14:16–20
Tada S et al (2011) Deleterious effects of lymphocytes at the early stage of neurodegeneration in an animal model of amyotrophic lateral sclerosis. J Neuroinflammation 8(1):19
Takeuchi S et al (2010) Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. J Neuropathol Exp Neurol 69(10):1044–1056
Tasaki Y et al (2012) Meloxicam ameliorates motor dysfunction and dopaminergic neurodegeneration by maintaining Akt-signaling in a mouse Parkinson’s disease model. Neurosci Lett 521(1):15–19
Thom SR et al (2008) Actin S-nitrosylation inhibits neutrophil beta2 integrin function. J Biol Chem 283(16):10822–10834
Thom SR et al (2013) Nitric-oxide synthase-2 linkage to focal adhesion kinase in neutrophils influences enzyme activity and β2 integrin function. J Biol Chem 288(7):4810–4818
Toohey JI (2012) The conversion of H2S to sulfane sulfur. Nat Rev Mol Cell Biol 13(12):803. doi:10.1038/nrm3391-c1
Townsend DM et al (2009) Novel role for glutathione S-transferase pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J Biol Chem 284(1):436–445
Träger U, Tabrizi SJ (2013) Peripheral inflammation in neurodegeneration. J Mol Med (Berlin, Germany) 91(6):673–681
Turner MR et al (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 15(3):601–609
Urushitani M, Ezzi SA, Julien J-P (2007) Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 104(7):2495–2500
Walden H, Martinez-Torres RJ (2012) Regulation of Parkin E3 ubiquitin ligase activity. Cel Mol Life Sci 69(18):3053–3067
Wang J et al (2001) Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem 276(51):47763–47766
Wang J, Pan S, Berk BC (2007) Glutaredoxin mediates Akt and eNOS activation by flow in a glutathione reductase-dependent manner. Arterioscler Thromb Vasc Biol 27(6):1283–1288
Wild E et al (2011) Abnormal peripheral chemokine profile in Huntington’s disease. PLoS Curr Hungtington Dis 1:1–9
Winkler BS, Orselli SM, Rex TS (1994) The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med 17(4):333–349
Wu DC et al (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22(5):1763–1771
Xiao Q et al (2007) Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem 102(6):2008–2019
Xing K-Y, Lou MF (2010) Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Investig Ophthalmol Vis Sci 51(12):6598–6604
Yang H et al (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93(6):865–873
Yazdi AS et al (2010) Inflammatory caspases in innate immunity and inflammation. J Innate Immun 2(3):228–237
Yu C, Li S, Whorton AR (2005) Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol 68(3):847–854
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809
Yu Y, Smoligovets AA, Groves JT (2013) Modulation of T cell signaling by the actin cytoskeleton. J Cell Sci 126(Pt 5):1049–1058
Zhang Y et al (2013) Kinase AKT controls innate immune cell development and function. Immunology 140(2):143–152
Zhang D et al (2014) Detection of protein S-sulfhydration by a tag-switch technique. Angewandte Chemie (International ed. in English) 53(2):575–581
Zhao W et al (2010) Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia 58(2):231–243
Acknowledgments
We thank Clinton J. Miller, Michael E. Maguire, and George Dubyak for critical reading of manuscript prior to submission. This work was supported in part by Department of Veterans Affairs (merit review Grant BX000290 to J.J.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorelenkova Miller, O., Mieyal, J.J. Sulfhydryl-mediated redox signaling in inflammation: role in neurodegenerative diseases. Arch Toxicol 89, 1439–1467 (2015). https://doi.org/10.1007/s00204-015-1496-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1496-7