Abstract
Summary
FRAX-based cost-effective intervention thresholds in the Swiss setting were determined. Assuming a willingness to pay at 2× Gross Domestic Product per capita, an intervention aimed at reducing fracture risk in women and men with a 10-year probability for a major osteoporotic fracture at or above 15% is cost-effective.
Introduction
The fracture risk assessment algorithm FRAX® has been recently calibrated for Switzerland. The aim of the present analysis was to determine FRAX-based fracture probabilities at which intervention becomes cost-effective.
Methods
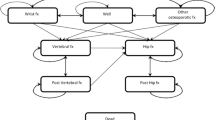
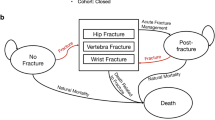
A previously developed and validated state transition Markov cohort model was populated with Swiss epidemiological and cost input parameters. Cost-effective FRAX-based intervention thresholds (cost-effectiveness approach) and the cost-effectiveness of intervention with alendronate (original molecule) in subjects with a FRAX-based fracture risk equivalent to that of a woman with a prior fragility fracture and no other risk factor (translational approach) were calculated based on the Swiss FRAX model and assuming a willingness to pay of 2 times Gross Domestic Product per capita for one Quality-adjusted Life-Year.
Results
In Swiss women and men aged 50 years and older, drug intervention aimed at decreasing fracture risk was cost-effective with a 10-year probability for a major osteoporotic fracture at or above 13.8% (range 10.8% to 15.0%) and 15.1% (range 9.9% to 19.9%), respectively. Age-dependent variations around these mean values were modest. Using the translational approach, treatment was cost-effective or cost-saving after the age 60 years in women and 55 in men who had previously sustained a fragility fracture. Using the latter approach leads to considerable underuse of the current potential for cost-effective interventions against fractures.
Conclusions
Using a FRAX-based intervention threshold of 15% for both women and men should permit cost-effective access to therapy to patients at high fracture probability based on clinical risk factors and thereby contribute to further reduce the growing burden of osteoporotic fractures in Switzerland.




Similar content being viewed by others
References
Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428
Lippuner K, Golder M, Greiner R (2005) Epidemiology and direct medical costs of osteoporotic fractures in men and women in Switzerland. Osteoporos Int 16(Suppl 2):S8–S17
Lippuner K, Johansson H, Kanis JA, Rizzoli R (2009) Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int 20:1131–1140
Robine JM, Paccaud F (2005) Nonagenarians and centenarians in Switzerland, 1860–2001: a demographic analysis. J Epidemiol Community Health 59:31–37
Bundesamt für Statistik. Szenarien zur Bevölkerungsentwicklung der Schweiz 2005–2050. http://www.bfs.admin.ch/bfs/portal/de/index/news/publikationen.Document.83713.pdf. Last visited May 14, 2008.
Schwenkglenks M, Lippuner K, Hauselmann HJ, Szucs TD (2005) A model of osteoporosis impact in Switzerland 2000–2020. Osteoporos Int 16:659–671
(1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94(6):646–650
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Cummings SR, Black D (1995) Bone mass measurements and risk of fracture in Caucasian women: a review of findings from prospective studies. Am J Med 98:24S–28S
(2011) Bundesamt für Gesundheit (BAG). List of reimbursed medicines in Switzerland (Spezialitätenliste). http://www.sl.bag.admin.ch
Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A (2000) Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 27:585–590
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822
Suhm N, Lamy O, Lippuner K (2008) Management of fragility fractures in Switzerland: results of a nationwide survey. Swiss Med Wkly 138(45–46):674–683
Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R (2002) An osteoporosis clinical pathway for the medical management of patients with low-trauma fracture. Osteoporos Int 13:450–455
Lippuner K, Johansson H, Kanis JA, Rizzoli R (2010) FRAX assessment of osteoporotic fracture probability in Switzerland. Osteoporos Int 21:381–389
Kanis JA, Johansson H, Oden A, McCloskey EV (2009) Assessment of fracture risk. Eur J Radiol 71:392–397
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A (2008) Case finding for the management of osteoporosis with FRAX assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408
Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, Baim S, Favus MJ, Khosla S, Lindsay RL (2008) Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int 19:449–458
Tosteson AN, Melton LJ 3rd, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL (2008) Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 19:437–447
Dawson-Hughes B (2008) A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab 93:2463–2465
Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 62:105–108
(2010) National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/professionals/clinical-guidelines.
Papaioannou A, Morin S, Cheung AM et al (2010) 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Cmaj 182:1864–1873
(1999) Royal College of Physicians. Osteoporosis: clinical guidelines for the prevention and treatment. London, Royal College of Physicians
(2010) Diagnostik, Prävention und Behandlung der Osteoporose: Empfehlungen der Schweizerischen Gesellschaft gegen Osteoporose (SVGO). http://www.svgo.ch/. Last visited August 8, 2011:
Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E (2009) FRAX and its applications to clinical practice. Bone 44:734–743
Kanis J (2007) World Health Organization Scientific Group. Assessment of osteoporosis at the primary health care level. Technical report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK.
Kanis JA, Oden A, Johnell O et al (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046
Krieg MA, Cornuz J, Ruffieux C et al (2006) Prediction of hip fracture risk by quantitative ultrasound in more than 7000 Swiss women > or =70 years of age: comparison of three technologically different bone ultrasound devices in the SEMOF study. J Bone Miner Res 21:1457–1463
Kind P, Dolan P, Gudex C, Williams A (1998) Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 316:736–741
Borgstrom F, Zethraeus N, Johnell O et al (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17:637–650
Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, Jonsson B (2004) The risk and burden of vertebral fractures in Sweden. Osteoporos Int 15:20–26
Kanis JA, Adams J, Borgstrom F, Cooper C, Jonsson B, Preedy D, Selby P, Compston J (2008) The cost-effectiveness of alendronate in the management of osteoporosis. Bone 42:4–15
Kanis JA, Borgstrom F, Zethraeus N, Johnell O, Oden A, Jonsson B (2005) Intervention thresholds for osteoporosis in the UK. Bone 36:22–32
Kanis JA, Johnell O, Oden A, Borgstrom F, Johansson H, De Laet C, Jonsson B (2005) Intervention thresholds for osteoporosis in men and women: a study based on data from Sweden. Osteoporos Int 16:6–14
Zethraeus N, Borgstrom F, Strom O, Kanis JA, Jonsson B (2007) Cost-effectiveness of the treatment and prevention of osteoporosis—a review of the literature and a reference model. Osteoporos Int 18:9–23
Borgstrom F, Strom O, Coelho J, Johansson H, Oden A, McCloskey EV, Kanis JA (2010) The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the FRAX. Osteoporos Int 21:495–505
Borgstrom F, Strom O, Coelho J, Johansson H, Oden A, McCloskey E, Kanis JA (2010) The cost-effectiveness of strontium ranelate in the UK for the management of osteoporosis. Osteoporos Int 21:339–349
Borgstrom F, Carlsson A, Sintonen H, Boonen S, Haentjens P, Burge R, Johnell O, Jonsson B, Kanis JA (2006) The cost-effectiveness of risedronate in the treatment of osteoporosis: an international perspective. Osteoporos Int 17:996–1007
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11:669–674
(2008) Life tables for WHO member states. Switzerland. <http://www.who.int/healthinfo/statistics/mortality_life_tables/en/> Accessed May 12, 2011.
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Oden A, Dawson A, Dere W, Johnell O, Jonsson B, Kanis JA (1998) Lifetime risk of hip fractures is underestimated. Osteoporos Int 8:599–603
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15:108–112
Poor G, Atkinson EJ, O’Fallon WM, Melton LJ 3rd (1995) Determinants of reduced survival following hip fractures in men. Clin Orthop Relat Res (319):260–265
Parker MJ, Anand JK (1991) What is the true mortality of hip fractures? Public Health 105:443–446
(2008) NICE. Osteoporosis—secondary prevention including strontium ranelate: appraisal consultation document. http://guidance.nice.org.uk/TA161 under www.nice.org.uk. Accessed May 12, 2011.
Jonsson B, Strom O, Eisman JA, Papaioannou A, Siris ES, Tosteson A, Kanis JA (2011) Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 22:967–982
(2008) National Institute for Health and Clinical Excellence (NICE). Systematic reviews of clinical effectiveness prepared for the guideline ‘Osteoporosis: assessment of fracture risk and the prevention of osteoporotic fractures in individuals at high risk’ http://www.nice.org.uk/nicemedia/live/11621/42362/42362.pdf. Last visited April18, 2011.
Kanis JA, Borgstrom F, Johnell O, Jonsson B (2004) Cost-effectiveness of risedronate for the treatment of osteoporosis and prevention of fractures in postmenopausal women. Osteoporos Int 15:862–871
Kanis JA, Borgstrom F, Johnell O, Oden A, Sykes D, Jonsson B (2005) Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporos Int 16:15–25
Schwenkglenks M, Lippuner K (2007) Simulation-based cost-utility analysis of population screening-based alendronate use in Switzerland. Osteoporos Int 18:1481–1491
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Black DM, Schwartz AV, Ensrud KE et al (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C (2003) Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone 33:301–307
Lloyd Jones M, Wilkinson A (2006) Adverse effects and persistence with therapy in patients taking oral alendronate, etidronate or risedronate: a systematic review. NHS R & D HTA ScHARR. http://www.nice.org.uk/nicemedia/live/11680/36718/36718.pdf. Accessed May 12, 2011.
(2011) OECD Consumer Price Indices. Available from www.oecd.org/std/prices-indices. http://stats.oecd.org/Index.aspx?querytype=view&queryname=221. Accessed May 12, 2011.
Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R (2002) Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int 13:731–737
Suhm N, Lamy O, Lippuner K (2008) Management of fragility fractures in Switzerland: results of a nationwide survey. Swiss Med Wkly 138:674–683
Szucs TD, Hauselmann H (2000) Die Wirtschaftlichkeit von Alendronat in der Behandlung der postmenopausalen Osteoporose. Ökon Qual Manag 5:99–106
(2001) Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. Available under http://whqlibdoc.who.int/publications/2001/924154550x.pdf. Last accessed October 4th, 2011. Geneva, World Health Organization
Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B (2004) Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 7:518–528
Borgstrom F, Johnell O, Kanis JA, Jonsson B, Rehnberg C (2006) At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 17:1459–1471
Swiss Federal Statistical Office (SFSO). Gross Domestic Product per capita. http://www.bfs.admin.ch/bfs/portal/de/index/themen/04/02/01/key/bip_einw.html. Last visited April 18th, 2011.
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164:1108–1112
Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA (2006) The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 17:1404–1409
Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, Seeman E (2006) Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone 38:694–700
Kanis JA, Mc Closkey E, Jonsson B, Cooper A, Strom O, Borgstrom F (2010) An evaluation of the NICE guidance for the prevention of osteoporotic fragility fractures in postmenopausal women. Archives of Osteoporosis 5:19–48
Kanis JA, Reginster JY, Kaufman JM, Ringe JD, Adachi JD, Hiligsmann M, Rizzoli R, Cooper C (2011) A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int (in press)
Ringe JD, Moller G (2009) Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol Int 30:213–221
Sheehy O, Kindundu CM, Barbeau M, LeLorier J (2009) Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int 20:1369–1376
Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R (2011) Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int 22:2565–2573
Kanis JA, Jonsson B (2002) Economic evaluation of interventions for osteoporosis. Osteoporos Int 13:765–767
McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, Jalava T, Kanis JA (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy—additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
Kanis JA, Johansson H, Oden A, McCloskey EV (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054
Kanis JA, Johansson H, Oden A, McCloskey EV (2010) A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone 47:729–735
McCloskey E, Lewiecki EM, Kanis JA et al (2011) Denosumab reduces the risk of clinical osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX®. Osteoporos Int 22(supplement 1):S103
Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD (2011) FRAX® with and without BMD. Calcif Tissue Int (in press)
Lippuner K, Popp AW, Schwab P, Gitlin M, Schaufler T, Senn C, Perrelet R (2010) Fracture hospitalizations between years 2000 and 2007 in Switzerland: a trend analysis. Osteoporos Int (in press)
Chevalley T, Guilley E, Herrmann FR, Hoffmeyer P, Rapin CH, Rizzoli R (2007) Incidence of hip fracture over a 10-year period (1991–2000): reversal of a secular trend. Bone 40:1284–1289
Acknowledgements
We are grateful to Martin Kleman, Innovus, Stockholm, Sweden for his contribution to health economic modelling and running the simulations and to Dr. Philippe Kress, Kressmed, Glattbrugg, Switzerland for his contribution to data analysis and his critical review of the manuscript.
Conflicts of interest
None
Disclaimers
None
Funding
This work was supported by an unrestricted research grant from MSD Switzerland AG. The sponsor had no influence on design, analysis, or interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lippuner, K., Johansson, H., Borgström, F. et al. Cost-effective intervention thresholds against osteoporotic fractures based on FRAX® in Switzerland. Osteoporos Int 23, 2579–2589 (2012). https://doi.org/10.1007/s00198-011-1869-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1869-6