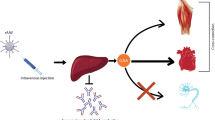
Abstract
Pompe disease is characterized by accumulation of both lysosomal and cytoplasmic glycogen primarily in skeletal and cardiac muscles. Mannose-6-phosphate receptor-mediated enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (rhGAA) targets the enzyme to lysosomes and thus is unable to digest cytoplasmic glycogen. Studies have shown that anti-DNA antibody 3E10 penetrates living cells and delivers “cargo” proteins to the cytosol or nucleus via equilibrative nucleoside transporter ENT2. We speculate that 3E10-mediated ERT with GAA will target both lysosomal and cytoplasmic glycogen in Pompe disease. A fusion protein (FabGAA) containing a humanized Fab fragment derived from the murine 3E10 antibody and the 110 kDa human GAA precursor was constructed and produced in CHO cells. Immunostaining with an anti-Fab antibody revealed that the Fab signals did not co-localize with the lysosomal marker LAMP2 in cultured L6 myoblasts or Pompe patient fibroblasts after incubation with FabGAA. Western blot with an anti-GAA antibody showed presence of the 150 kDa full-length FabGAA in the cell lysates, in addition to the 95- and 76 kDa processed forms of GAA that were also seen in the rhGAA-treated cells. Blocking of mannose-6-phosphate receptor with mannose-6-phosphate markedly reduced the 95- and the 76 kDa forms but not the 150 kDa form. In GAA-KO mice, FabGAA achieved similar treatment efficacy as rhGAA at an equal molar dose in reducing tissue glycogen contents. Our data suggest that FabGAA retains the ability of rhGAA to treat lysosomal glycogen accumulation and has the beneficial potential over rhGAA to reduce cytoplasmic glycogen storage in Pompe disease.
Key messages
-
FabGAA can be delivered to both the cytoplasm and lysosomes in cultured cells.
-
FabGAA equally reduced lysosomal glycogen accumulation as rhGAA in GAA-KO mice.
-
FabGAA has the beneficial potential over rhGAA to clear cytoplasmic glycogen.
-
This study suggests a novel antibody-enzyme fusion protein therapy for Pompe disease.






Similar content being viewed by others
References
van der Ploeg AT, Reuser AJ (2008) Pompe’s disease. Lancet 372(9646):1342–1353
Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D (2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148(5):671–676
Muller-Felber W, Horvath R, Gempel K, Podskarbi T, Shin Y, Pongratz D, Walter MC, Baethmann M, Schlotter-Weigel B, Lochmuller H et al (2007) Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord 17(9–10):698–706
Kishnani PS, Beckemeyer AA, Mendelsohn NJ (2012) The new era of Pompe disease: advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management. Am J Med Genet C Semin Med Genet 160(1):1–7
Angelini C, Semplicini C (2012) Enzyme replacement therapy for Pompe disease. Curr Neurol Neurosci Rep 12(1):70–75
Case LE, Beckemeyer AA, Kishnani PS (2012) Infantile Pompe disease on ERT: update on clinical presentation, musculoskeletal management, and exercise considerations. Am J Med Genet C Semin Med Genet 160(1):69–79
Van Hove JL, Yang HW, Wu JY, Brady RO, Chen YT (1996) High-level production of recombinant human lysosomal acid alpha-glucosidase in Chinese hamster ovary cells which targets to heart muscle and corrects glycogen accumulation in fibroblasts from patients with Pompe disease. Proc Natl Acad Sci U S A 93(1):65–70
Garancis JC (1968) Type II glycogenosis. Biochemical and electron microscopic study. Am J Med 44(2):289–300
Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, Kishnani PS, O’Callaghan M (2006) Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest 86(12):1208–1220
Lewandowska E, Wierzba-Bobrowicz T, Rola R, Modzelewska J, Stepien T, Lugowska A, Pasennik E, Ryglewicz D (2008) Pathology of skeletal muscle cells in adult-onset glycogenosis type II (Pompe disease): ultrastructural study. Folia Neuropathol 46(2):123–133
Griffin JL (1984) Infantile acid maltase deficiency. I. Muscle fiber destruction after lysosomal rupture. Virchows Arch B Cell Pathol Incl Mol Pathol 45(1):23–36
Rehman K, Hamid Akash MS, Akhtar B, Tariq M, Mahmood A, Ibrahim M (2016) Delivery of therapeutic proteins: challenges and strategies. Curr Drug Targets 17(10):1172–1188
Komin A, Russell LM, Hristova KA, Searson PC (2016) Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: mechanisms and challenges. Adv Drug Deliv Rev. doi:10.1016/j.addr.2016.06.002
Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH (1996) Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J Immunol 157(5):2082–2088
Hansen JE, Sohn W, Kim C, Chang SS, Huang NC, Santos DG, Chan G, Weisbart RH, Nishimura RN (2006) Antibody-mediated Hsp70 protein therapy. Brain Res 1088(1):187–196
Weisbart RH, Stempniak M, Harris S, Zack DJ, Ferreri K (1998) An autoantibody is modified for use as a delivery system to target the cell nucleus: therapeutic implications. J Autoimmun 11(5):539–546
Lawlor MW, Armstrong D, Viola MG, Widrick JJ, Meng H, Grange RW, Childers MK, Hsu CP, O’Callaghan M, Pierson CR et al (2013) Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Hum Mol Genet 22(8):1525–1538
Hansen JE, Tse CM, Chan G, Heinze ER, Nishimura RN, Weisbart RH (2007) Intranuclear protein transduction through a nucleoside salvage pathway. J Biol Chem 282(29):20790–20793
Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR (2001) Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem Biophys Res Commun 280(3):951–959
Lu H, Chen C, Klaassen C (2004) Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug Metab Dispos 32(12):1455–1461
Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B et al (1998) Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 273(30):19086–19092
Maga JA, Zhou J, Kambampati R, Peng S, Wang X, Bohnsack RN, Thomm A, Golata S, Tom P, Dahms NM et al (2013) Glycosylation-independent lysosomal targeting of acid alpha-glucosidase enhances muscle glycogen clearance in Pompe mice. J Biol Chem 288(3):1428–1438
Joseph A, Munroe K, Housman M, Garman R, Richards S (2008) Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol 152(1):138–146
Hallgren P, Hansson G, Henriksson KG, Hager A, Lundblad A, Svensson S (1974) Increased excretion of a glucose-containing tetrasaccharide in the urine of a patient with glycogen storage disease type II (Pompe’s disease). Eur J Clin Invest 4(6):429–433
Yi H, Thurberg BL, Curtis S, Austin S, Fyfe J, Koeberl DD, Kishnani PS, Sun B (2012) Characterization of a canine model of glycogen storage disease type IIIa. Dis Model Mech 5(6):804–811
Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, Chen YT (1999) Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A 96(16):8861–8866
Young SP, Stevens RD, An Y, Chen YT, Millington DS (2003) Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution-electrospray ionization tandem mass spectrometry. Anal Biochem 316(2):175–180
Yi H, Fredrickson KB, Das S, Kishnani PS, Sun B (2013) Stbd1 is highly elevated in skeletal muscle of Pompe disease mice but suppression of its expression does not affect lysosomal glycogen accumulation. Mol Genet Metab 109(3):312–314
Moreland RJ, Jin X, Zhang XK, Decker RW, Albee KL, Lee KL, Cauthron RD, Brewer K, Edmunds T, Canfield WM (2005) Lysosomal acid alpha-glucosidase consists of four different peptides processed from a single chain precursor. J Biol Chem 280(8):6780–6791
Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, Mattaliano RJ, Nagaraju K, Plotz PH (2003) Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 80(1–2):159–169
Raben N, Jatkar T, Lee A, Lu N, Dwivedi S, Nagaraju K, Plotz PH (2002) Glycogen stored in skeletal but not in cardiac muscle in acid alpha-glucosidase mutant (Pompe) mice is highly resistant to transgene-encoded human enzyme. Mol Ther 6(5):601–608
Sun B, Zhang H, Bird A, Li S, Young SP, Koeberl DD (2009) Impaired clearance of accumulated lysosomal glycogen in advanced Pompe disease despite high-level vector-mediated transgene expression. J Gene Med 11(10):913–920
Stewart KM, Horton KL, Kelley SO (2008) Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem 6(13):2242–2255
Skotland T, Iversen TG, Torgersen ML, Sandvig K (2015) Cell-penetrating peptides: possibilities and challenges for drug delivery in vitro and in vivo. Molecules 20(7):13313–13323
Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, Altmann A, Haberkorn U, Mier W (2010) The pharmacokinetics of cell-penetrating peptides. Mol Pharm 7(6):2224–2231
Acknowledgements
This work was supported by a grant from Valerion Therapeutics (Concord, MA). We thank Dr. Tracy R. McKnight for reviewing and editing the manuscript. We also thank Ms. Fengqin Gao for her assistance with animal care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Armstrong is the founder of Valerion Therapeutics and declares ownership interest in the company. All other authors declare no competing or financial interests.
Additional information
Haiqing Yi and Tao Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yi, H., Sun, T., Armstrong, D. et al. Antibody-mediated enzyme replacement therapy targeting both lysosomal and cytoplasmic glycogen in Pompe disease. J Mol Med 95, 513–521 (2017). https://doi.org/10.1007/s00109-017-1505-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1505-9