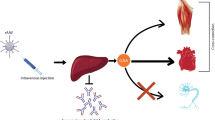
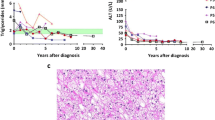
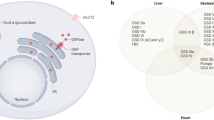
Abstract
Late-onset glycogenosis type II (glycogen storage disease type II [GSDII]) is a rare autosomal disorder caused by deficiency of acid maltase, a lysosomal enzyme that hydrolyzes glycogen to glucose. Recently, both infantile and adult GSDII patients have been treated with enzyme replacement therapy (ERT), and a number of studies including large cohorts of GSDII patients have recently demonstrated that ERT is effective in modifying the natural course of the disease. The opportunity of this new treatment gave new hope to patients, but also an important impulse to the research on every feature of the disease, leading to a deeper knowledge on the response to treatment, on clinical manifestations, and on pathophysiologic aspects such as the role of autophagy and immune status.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Angelini C, Engel AG. Subcellular distribution of acid and neutral alpha-glucosidases in normal, acid maltase deficient, and myophosphorylase deficient human skeletal muscle. Arch Biochem Biophys. 1973;156:350–5.
Hirschhorn R, Reuser, AJ. Glycogen storage disease type II: acid alphaglucosidase (acid maltase) deficiency. In: CR Scriver BA, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. p. 3389–420
van der Ploeg AT, Reuser AJ. Pompe’s disease. Lancet. 2008;372:1342–53.
Bembi B, Cerini E, Danesino C, et al. Diagnosis of glycogenosis type II. Neurology. 2008;71:S4–S11.
Laforet P, Nicolino M, Eymard PB, et al. Juvenile and adult onset acid maltase deficiency in France: genotype-phenotype correlation. Neurology. 2000;55:1122–8.
Kishnani PS, Hwu WL, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–6.
Hagemans MLC, Winkel LP, Hop WC, et al. Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 2005;64:2139–214.
Mellies U, Stehling F, Dohna-Schwake C, et al. Respiratory failure in Pompe disease: treatment with noninvasive ventilation. Neurology. 2005;64:1465–7.
Hagemans ML, Hop WJ, Van Doorn PA, et al. Course of disability and respiratory function in untreated lae onset Pompe disease. Neurology. 2006;66:581–3.
Angelini C, Semplicini C, Ravaglia S, and the Italian GSDII Group. Long-term follow-up effects on enzyme replacement treatment of adult form of acid maltase deficiency myopathy. Acta Myologica. 2011; in Press.
Hagemans MLC, Janssens ACJW, Winkel LPF, et al. Late-onset Pompe disease primarily affects quality of life in physical health domains. Neurology. 2004;63:1688–92.
Gungor D, de Vries JM, Hop WCJ, et al. Survival and associated factors in 268 adults with Pompe disease prior to treatment with enzyme replacement therapy. Orphanet J Rare Dis. 2011;6:34.
Kanters TA, Hagemans ML, van der Beek NA, et al. Burden of illness of Pompe disease in patients only receiving supportive care. Inherit Metab Dis. 2011 Apr 16. [Epub ahead of print].
Nascimbeni AC, Fanin M, Tasca E, Angelini C. Molecular pathology and enzyme processing in various phenotypes of acid maltase deficiency. Neurology. 2008;70:617–26.
Hug G, Schubert WK. Lysosomes in type II glycogenosis. Changes during administration of extract from Aspergillus niger. J Cell Biol. 1967;35:C1–6.
Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–30.
Neufeld EF. Enzyme replacement therapy – a brief history. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry disease: perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 10.
Rossi M, Parenti G, Della Casa R, et al. Long-term enzyme replacement therapy for pompe disease with recombinant human alpha-glucosidase derived from chinese hamster ovary cells. J Child Neurol. 2007;22:565–73.
Van den Hout H, Reuser AJ, Vulto AG, et al. Recombinant human alpha-glucosidase from rabbit milk in Pompe patients. Lancet. 2000;356:397–8.
Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109.
Kishnani PS, Nicolino M, Voit T, et al. Results from a phase II trial of Chinese hamster ovary cell-derived recombinant human acid a-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97.
van der Ploeg AT. Where do we stand in enzyme replacement therapy in Pompe’s disease? Neuromuscul Disord. 2010;20(12):773–4.
• Bembi B, Cerini E, Danesino C, et al. Management and treatment of glycogenosis type II. Neurology. 2008;71:S12–36. A task force of physicians reviewed the literature to provide guidelines for clinical and instrumental follow-up of GSDII patients, to monitor the natural course of the disease and to prevent and/or treat early any complication of this multisystemic disorder.
•• van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362(15):1396–406. This is the only double-blind randomized placebo-controlled study on efficacy of ERT in late onset patients. It demonstrated the efficacy of treatment in ameliorating motor and respiratory function.
Strothotte S, Strigi-Pill N, Grunert B, et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol. 2010;257:91–7.
Bembi B, Pisa FE, Confalonieri M, et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J Inherit Metab Dis. 2010;33:727–35.
van Capelle CI, Winkel LP, Hagemans ML, et al. Eight years experience with enzyme replacement therapy in two children and one adult with Pompe disease. Neuromuscul Disord. 2008;18:447–52.
Kobayashi H, Shimada Y, Ikegami M, et al. Prognostic factors for the late onset Pompe disease with enzyme replacement therapy: from our experience of 4 cases including an autopsy case. Mol Genet Metab. 2010;100:14–9.
Ravaglia S, Carlucci A, Danesino C. Prognostic factors for late-onset Pompe disease with enzyme replacement therapy: the two sides of low BMI. Mol Genet Metab. 2010;100:388.
Orlikowski D, Pellegrini N, Prigent H, et al. Recombinant human acid alpha-glucosidase (rhGAA) in adult patients with severe respiratory failure due to Pompe disease. Neuromuscul Disord. 2011;21:477–82.
Drost MR, Schaart G, van Dijk P, et al. Both type 1 and type 2a muscle fibers can respond to enzyme therapy in Pompe disease. Muscle Nerve. 2008;37(2):251–5.
Drost MR, Hesselink RP, Oomens CW, van der Vusse GJ. Effects of non contractile inclusions on mechanical performance of skeletal muscle. J Biomech. 2005;38:1035–43.
• Raben N, Takikita S, Pittis MG, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand.. Autophagy. 2007;3:546–52. This paper reviews the importance of autophagy in pathophysiology of GSDII and reveals new mechanisms involved in the disease.
Thurberg BL, Lynch Maloney C, Vaccaro C, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86:1208–20.
Raben N, Ralston E, Chien YH, et al. Differences in the predominance of lysosomal and autophagic pathologies between infants and adults with Pompe disease: implications for therapy. Mol Genet Metab. 2010;101:324–31.
Raben N, Schreiner C, Baum R, et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder–murine Pompe disease. Autophagy. 2010;6:1078–89.
Fukuda T, Roberts A, Ahearn M, et al. Autophagy and lysosomes in Pompe disease. Autophagy. 2006;2:318–20.
Zampieri S, Buratti E, Dominissini S, et al. Splicing mutations in glycogen-storage disease type II: evaluation of the full spectrum of mutations and their relation to patients’ phenotypes. Eur J Hum Genet. 2011;19:422–31.
Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, Oostra BA. Characterization of the human lysosomal alpha-glucosidase gene. Biochem J. 1990;272:493–7.
Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33.
de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab. 2010;101:338–45.
Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: Lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–36.
Joseph A, Munroe K, Housman M, et al. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol. 2008;152:138–46.
Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360:194–5.
Koeberl DD, Kishnani PS. Immunomodulatory gene therapy in lysosomal storage disorders. Curr Gene Ther. 2009;9(6):503–10.
Little JP, Phillips SM. Resistance exercise and nutrition to counteract muscle wasting. Appl Physiol Nutr Metab. 2009;34(5):817–28.
Slonim AE, Bulone L, Goldberg T, et al. Modification of the natural history of adult-onset acid maltase deficiency by nutrition and exercise therapy. Muscle Nerve. 2007;35:70–7.
Terzis G, Dimopoulos F, Papadimas GK, et al. Effect of aerobic and resistance exercise training on late-onset Pompe disease patients receiving enzyme replacement therapy. Mol Genet Metab. 2011 May 19. [Epub ahead of print].
Levenson D. Late onset Pompe disease revealed by newborn screening. Am J Med Genet A. 2011;155A(5).
• Chien YH, Lee NC, Huang HJ, et al. Later-onset pompe disease: early detection and early treatment initiation enabled by newborn screening. J Pediatr. 2011;158:1023–7. This paper discusses the importance of early diagnosis with newborn screening in metabolic myopathies such as GSDII, which may change more significantly the natural course of the disease.
Zhu Y, Jiang JL, Gumlaw NK, et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol Ther. 2009;17:954–63.
Flanagan JJ, Rossi B, Tang K, et al. The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum Mutat. 2009;30:1683–92.
Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med. 2009;1:268–79.
Acknowledgment
This paper was supported by Telethon GUP-007001, EuroBioBank, and TREAT-NMD.
Disclosure
Conflicts of interest: C. Angelini: received travel/accommodations expenses covered or reimbursed for IV Steps Forward in Pompe Disease; C. Semplicini: received an honorarium for a speech at IV Steps Forward in Pompe Disease.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angelini, C., Semplicini, C. Enzyme Replacement Therapy for Pompe Disease. Curr Neurol Neurosci Rep 12, 70–75 (2012). https://doi.org/10.1007/s11910-011-0236-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-011-0236-5