Abstract
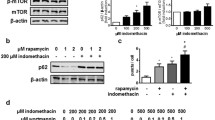
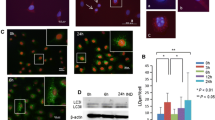
Non-steroidal anti-inflammatory drug (NSAID)-associated endoplasmic reticulum (ER) stress (a cyclooxygenase-2-independent mechanism) and consequent autophagic cell death are responsible for NSAID-associated gastric damage. Therefore, alleviating cytotoxicity executed via ER stress and autophagy can be a strategy to prevent NSAID-associated gastric damage. Here, we explored whether genetic or pharmacologic inhibition of autophagy can mitigate NSAID-associated gastric damage in in vitro and in vivo models. To examine the effects of genetic inhibition of NSAID-associated autophagy, we administered indomethacin to RGM1 gastric mucosal cells transfected with shPERK, siLC3B, or shATG5 and microtubule-associated protein light chain 3B knock-out (LC3B−/−) mice. 3-Methyladenine (3-MA) or chloroquine (CQ) was used for pharmacologic inhibition of autophagy in both models. Indomethacin administration increased the expression of ER stress proteins including GRP78, ATF6, and CHOP. Indomethacin provoked the appearance of autophagic vesicles with the increased expression of ATG5 and LC3B-II. Genetic ablation of various ER stress genes significantly attenuated indomethacin-induced autophagy and apoptosis (p < 0.01), whereas knock-down of either ATG5 or LC3B significantly reduced indomethacin-induced cytotoxicity (p < 0.01). Testing each of the genes implicated in ER stress and autophagy showed that indomethacin leads to gastric cell apoptosis through autophagy induction consequent to ER stress. Pharmacological inhibition of autophagy with either 3-MA or CQ in rats or genetic ablation of LC3B in mice all had a significant rescuing effect against indomethacin-associated gastric damage (p < 0.01) and a decrease in molecular markers of autophagic and apoptotic gastric cells. In conclusion, preemptive autophagy inhibition can be a potential strategy to mitigate NSAID-associated gastric damage.
Key messages
-
NSAID administration triggered ER stress and subsequent autophagy.
-
Inhibition of autophagy resulted in attenuated NSAID-associated cytotoxicity.
-
Autophagy inhibitors represent a novel strategy to prevent NSAID-associated gastric damage.






Similar content being viewed by others
References
Graham DY, Chan FK (2008) NSAIDs, risks, and gastroprotective strategies: current status and future. Gastroenterology 134:1240–1246
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235
Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T (2004) Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ 11:1009–1016
Chen N, Karantza-Wadsworth V (2009) Role and regulation of autophagy in cancer. Biochim Biophys Acta 1793:1516–1523
Chen HY, White E (2011) Role of autophagy in cancer prevention. Cancer Prev Res (Phila) 4:973–983
Harada S, Nakagawa T, Yokoe S, Edogawa S, Takeuchi T, Inoue T, Higuchi K, Asahi M (2015) Autophagy deficiency diminishes indomethacin-induced intestinal epithelial cell damage through activation of the ERK/Nrf2/HO-1 pathway. J Pharmacol Exp Ther 355:353–361
Narabayashi K, Ito Y, Eid N, Maemura K, Inoue T, Takeuchi T, Otsuki Y, Higuchi K (2015) Indomethacin suppresses LAMP-2 expression and induces lipophagy and lipoapoptosis in rat enterocytes via the ER stress pathway. J Gastroenterol 50:541–554
Hernandez C, Barrachina MD, Vallecillo-Hernandez J, Alvarez A, Ortiz-Masia D, Cosin-Roger J, Esplugues JV, Calatayud S (2016) Aspirin-induced gastrointestinal damage is associated with an inhibition of epithelial cell autophagy. J Gastroenterol 51:691–701
Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM et al (2011) Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141:1314–1322 1322 e1311-1315
Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, Tully O, Kearney K, Lazowick D, Mercogliano G et al (2008) Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther 28:1317–1325
Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14:230–239
Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H (2009) Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol 16:761–771
Li J, Hou N, Faried A, Tsutsumi S, Kuwano H (2010) Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer 46:1900–1909
Szabo S, Trier JS, Brown A, Schnoor J, Homan HD, Bradford JC (1985) A quantitative method for assessing the extent of experimental gastric erosions and ulcers. J Pharmacol Methods 13:59–66
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Treiman M, Caspersen C, Christensen SB (1998) A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci 19:131–135
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 22:193–201
Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13:374–384
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595
Kondo Y, Kanzawa T, Sawaya R, Kondo S (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734
Moretti L, Attia A, Kim KW, Lu B (2007) Crosstalk between Bak/Bax and mTOR signaling regulates radiation-induced autophagy. Autophagy 3:142–144
Guillon-Munos A, van Bemmelen MX, Clarke PG (2006) Autophagy can be a killer even in apoptosis-competent cells. Autophagy 2:140–142
Xue L, Fletcher GC, Tolkovsky AM (1999) Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci 14:180–198
Chen ST, Thomas S, Gaffney KJ, Louie SG, Petasis NA, Schonthal AH (2010) Cytotoxic effects of celecoxib on Raji lymphoma cells correlate with aggravated endoplasmic reticulum stress but not with inhibition of cyclooxygenase-2. Leuk Res 34:250–253
Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schonthal AH (2008) Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Res 68:843–851
Pae HO, Jeong SO, Jeong GS, Kim KM, Kim HS, Kim SA, Kim YC, Kang SD, Kim BN, Chung HT (2007) Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem Biophys Res Commun 353:1040–1045
Chinta SJ, Poksay KS, Kaundinya G, Hart M, Bredesen DE, Andersen JK, Rao RV (2009) Endoplasmic reticulum stress-induced cell death in dopaminergic cells: effect of resveratrol. J Mol Neurosci 39:157–168
Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G (2008) To die or not to die: that is the autophagic question. Curr Mol Med 8:78–91
Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR (2012) New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 8:522–533
Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 17:654–666
Yang ZJ, Chee CE, Huang S, Sinicrope FA (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10:1533–1541
Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A (2012) Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8:200–212
Vazquez-Martin A, Lopez-Bonetc E, Cufi S, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, Menendez JA (2011) Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions. Drug Resist Updat 14:212–223
Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J et al (2012) Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J Biol Chem 287:12455–12468
Acknowledgements
The current study was granted by The Korean Society of Upper GI Disease and Helicobacter, by a National Research Foundation grant of Korea government (2010-0002052), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2058732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Chan Young Ock and Jong-Min Park contributed equally
Electronic supplementary material
ESM 1
(PDF 529 kb)
Rights and permissions
About this article
Cite this article
Ock, C.Y., Park, JM., Han, YM. et al. Genetic ablation or pharmacologic inhibition of autophagy mitigated NSAID-associated gastric damages. J Mol Med 95, 405–416 (2017). https://doi.org/10.1007/s00109-016-1491-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1491-3