Abstract
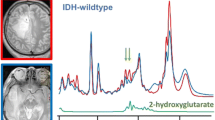
Somatic mutations in isocitrate dehydrogenase (IDH)1 and 2 have been identified in a subset of gliomas, rendering these tumors with elevated levels of “oncometabolite,” D-2-hydroxyglutarate (2HG). Herein, we report that 2HG can be precisely detected by magnetic resonance (MR) in human glioma specimens and used as a reliable biomarker to identify this subset of tumors. Specifically, we developed a two-dimensional correlation spectroscopy resonance method to reveal the distinctive cross-peak pattern of 2HG in the complex metabolite nuclear MR spectra of brain tumor tissues. This study demonstrates the feasibility, specificity, and selectivity of using MR detection and quantification of 2HG for the diagnosis and classification of IDH1/2 mutation-positive brain tumors. It further opens up the possibility of developing analogous non-invasive MR-based imaging and spectroscopy studies directly in humans in the neuro-oncology clinic.





Similar content being viewed by others
Notes
During our submission the following three related studies were published: Elkhaled A et al. (2012) Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med 4(116):116ra4; Andronesi OC et al. (2012) Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 4(116):116ra4; and Choi C et al. (2012) 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med doi:10.1038/nm.2682.
References
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60:166–193
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Hunter SB, Brat DJ, Olson JJ, Von Deimling A, Zhou W, Van Meir EG (2003) Alterations in molecular pathways of diffusely infiltrating glial neoplasms: application to tumor classification and anti-tumor therapy (Review). Int J Oncol 23:857–869
Tohma Y, Gratas C, Van Meir EG, Desbaillets I, Tenan M, Tachibana O, Kleihues P, Ohgaki H (1998) Necrogenesis and Fas/APO-1 (CD95) expression in primary (de novo) and secondary glioblastomas. J Neuropathol Exp Neurol 57:239–245
Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown PO et al (2005) Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A 102:5814–5819
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD et al (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361:1058–1066
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y et al (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324:261–265
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Xiao MT, Liu LX et al (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG (1997) Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 94:6408–6413
Morvan D, Demidem A, Papon J, De Latour M, Madelmont JC (2002) Melanoma tumors acquire a new phospholipid metabolism phenotype under cystemustine as revealed by high-resolution magic angle spinning proton nuclear magnetic resonance spectroscopy of intact tumor samples. Cancer Res 62:1890–1897
Andronesi OC, Becker S, Seidel K, Heise H, Young HS, Baldus M (2005) Determination of membrane protein structure and dynamics by magic-angle-spinning solid-state NMR spectroscopy. J Am Chem Soc 127:12965–12974
Mao H, Toufexis D, Wang X, Lacreuse A, Wu S (2007) Changes of metabolite profile in kainic acid induced hippocampal injury in rats measured by HRMAS NMR. Exp Brain Res 183:477–485
Shi C, Wang X, Wu S, Zhu Y, Chung LW, Mao H (2008) HRMAS 1H-NMR measured changes of the metabolite profile as mesenchymal stem cells differentiate to targeted fat cells in vitro: implications for non-invasive monitoring of stem cell differentiation in vivo. J Tissue Eng Regen Med 2:482–490
Rong Y, Belozerov VE, Tucker-Burden C, Chen G, Durden DL, Olson JJ, Van Meir EG, Mackman N, Brat DJ (2009) Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res 69:2540–2549
Chu H, Cole SR (2006) Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol 59:1331–1332, author reply 1332–1333
Sijens PE, Levendag PC, Vecht CJ, van Dijk P, Oudkerk M (1996) 1H MR spectroscopy detection of lipids and lactate in metastatic brain tumors. NMR Biomed 9:65–71
Li X, Vigneron DB, Cha S, Graves EE, Crawford F, Chang SM, Nelson SJ (2005) Relationship of MR-derived lactate, mobile lipids, and relative blood volume for gliomas in vivo. AJNR Am J Neuroradiol 26:760–769
Park I, Larson PE, Zierhut ML, Hu S, Bok R, Ozawa T, Kurhanewicz J, Vigneron DB, Vandenberg SR, James CD et al (2010) Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro Oncol 12:133–144
Glunde K, Jiang L, Moestue SA, Gribbestad IS (2011) MRS and MRSI guidance in molecular medicine: targeting and monitoring of choline and glucose metabolism in cancer. NMR Biomed 24:673–690
Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H et al (2012) Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol 107:197–205
Schubert F, Gallinat J, Seifert F, Rinneberg H (2004) Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. NeuroImage 21:1762–1771
Waddell KW, Avison MJ, Joers JM, Gore JC (2007) A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging 25:1032–1038
Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V et al (2007) Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med 58:236–244
Lymer K, Haga K, Marshall I, Sailasuta N, Wardlaw J (2007) Reproducibility of GABA measurements using 2D J-resolved magnetic resonance spectroscopy. Magn Reson Imaging 25:634–640
Li Y, Chen AP, Crane JC, Chang SM, Vigneron DB, Nelson SJ (2007) Three-dimensional J-resolved H-1 magnetic resonance spectroscopic imaging of volunteers and patients with brain tumors at 3 T. Magn Reson Med 58:886–892
Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A 108:3270–3275
Acknowledgments
We thank Dr. Jeffrey Olson for the provision of brain tumor samples, Drs. Daniel Brat and Steven Hunter for neuropathological diagnosis, and Narra Sarojini Devi and Zhaobin Zhang for banking brain tumor tissue samples. We also thank the Winship Cancer Institute Tissue Procurement and Pathology core for tissue sectioning and immunohistochemistry services.
Disclosure statement
All authors concur with the content of the manuscript and assert that this report is not under consideration for publication elsewhere. No conflict of interest is noted.
Grant support
This work was supported in part by NIH grants R01 CA86335 and CA116804 (to EGVM), R21AG032104-01A1 and P50CA128301-020003 (to HM), P30 CA138292 (to the Emory Winship Cancer Institute), NINDS Training Grant 2T32NS007480-11 (to JK and Allan I. Levey), a joint Translational Research Pilot Grant from the Winship Cancer Institute and the Atlanta Clinical & Translational Science Instite (ACTSI, UL1RR025008; to HM and EGVM), the Brain Tumor Funders Collaborative (to EGVM), and the Georgia Cancer Coalition (to JK and EGVM).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dr. Hui Mao and Dr. Erwin G. Van Meir are co-corresponding authors and have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Figure 1
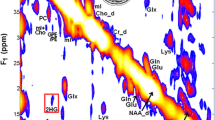
Stability analysis of 2HG in glioma samples during the HRMAS NMR analysis. a 2D COSY spectra of a representative sample (subject ID no: 0610) obtained from the first NMR experiment (left panel) and from a retest collected after 24 h (right panel) are shown. The cross-peaks from the 2HG unique J-coupling pattern (red box) and the resonances of Glx (blue box) are indicated. 2HG remains stable in glioma samples during sample re-analysis. Signal intensity color chart is shown. b The stability of a commercial 2HG preparation left for 7 days at 25°C was assessed by daily sampling and 1D NMR spectra. The unique 2HG cross-peaks are indicated. Note that the peaks marked by asterisk are impurities present in the commercial 95 % pure 2HG. (JPEG 139 kb)
Suppl. Figure 2
Calibration of 2HG levels in tumor samples analyzed by HRMAS NMR. The 2HG concentrations were calculated by measuring the peak integral of the selected 2HG protons calibrated with the known concentration of the internal reference, TSP, and from a dose-dependent reference spectrum. A dose-dependent standard curve of 2HG concentration as a function of the integral of 2HG resonance (α) is shown in a. 2D COSY spectra of a non-tumoral control without (b, left panel) or titrated with 2HG (b, right panel) confirm the cross-peaks’ assignment for 2HG. The cross-peaks from the 2HG unique J-coupling pattern (red box) and the resonances of Glx (blue box) are indicated. Signal intensity color chart is shown. (JPEG 95 kb)
Rights and permissions
About this article
Cite this article
Kalinina, J., Carroll, A., Wang, L. et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med 90, 1161–1171 (2012). https://doi.org/10.1007/s00109-012-0888-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0888-x