Abstract
Background
Mutations in the isocitrate dehydrogenase enzyme are present in a majority of lower-grade gliomas and secondary glioblastomas. This mis-sense mutation results in the neomorphic reduction of isocitrate dehydrogenase resulting in an accumulation of the “oncometabolite” 2-hydroxyglutarate (2HG). Detection of 2HG can thus serve as a surrogate biomarker for these mutations, with significant translational implications including improved prognostication. Two dimensional localized correlated spectroscopy (2D L-COSY) at 7T is a highly-sensitive non-invasive technique for assessing brain metabolism. This study aims to assess tumor metabolism using 2D L-COSY at 7T for the detection of 2HG in IDH-mutant gliomas.
Methods
Nine treatment-naïve patients with suspected intracranial neoplasms were scanned at 7T MRI/MRS scanner using the 2D L-COSY technique. 2D-spectral processing and analyses were performed using a MATLAB-based reconstruction algorithm. Cross and diagonal peak volumes were quantified in the 2D L-COSY spectra and normalized with respect to the creatine peak at 3.0 ppm and quantified data were compared with previously-published data from six normal subjects. Detection of 2HG was validated using findings from immunohistochemical (IHC) staining in patients who subsequently underwent surgical resection.
Results
2HG was detected in both of the IDH-mutated gliomas (grade III Anaplastic Astrocytoma and grade II Diffuse Astrocytoma) and was absent in IDH wild-type gliomas and in a patient with breast cancer metastases. 2D L-COSY was also able to resolve complex and overlapping resonances including phosphocholine (PC) from glycerophosphocholine (GPC), lactate (Lac) from lipids and glutamate (Glu) from glutamine (Gln).
Conclusions
This study demonstrates the ability of 2D L-COSY to unambiguously detect 2HG in addition to other neuro metabolites. These findings may aid in establishing 2HG as a biomarker of malignant progression as well as for disease monitoring in IDH-mutated gliomas.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Mutations in the gene encoding isocitrate dehydrogenase (IDH) occur in up to 80 % of World Health Organization (WHO) grade II/III gliomas and a majority of secondary glioblastomas (GBMs) [1–3]. Glioma patients, whose lesions harbor IDH mutations are associated with better prognosis, are more sensitive to chemo-radiation therapy and demonstrate longer survival than those with wild-type IDH [4, 5]. These mis-sense mutations may confer the neomorphic ability to reduce isocitrate dehydrogenase, resulting in the accumulation of the “oncometabolite” 2-hydroxyglutarate (2HG) [6, 7] rather than its normal catalysis into α-ketoglutarate. IDH-mutation status has taken on greater clinical significance in this era of molecular diagnostics, with recent major restructuring of central nervous system (CNS) tumors as published in the 2016 WHO classification [8].
In addition to 2HG, several other metabolic processes are altered in brain tumors. A prominent resonance from total choline comprising of free choline (Cho), phosphocholine (PC) and glycerophosphocholine (GPC) is commonly observed in conventional 1D proton magnetic resonance spectroscopy (1H MRS) studies of brain tumors. However, in order to properly understand the dysregulated Cho metabolism, it is important to detect and quantify relative levels of PC, a vital indicator for the expression and/or activity of choline kinase [9] and GPC. Also important is to understand the alteration in the glutamate (Glu), glutamine (Gln) and gamma-aminobutyric acid (GABA) cycle as Glu is involved in the cellular anabolic pathways and in facilitating tumor invasion [10].
Non-invasive detection of 2HG and other resonances on conventional 1H MRS is challenging due to extensive overlap with the resonances of neighboring metabolites. Previous 1H MRS studies have employed two different acquisition and processing strategies to resolve 2HG from overlapping resonances in vivo. Choi et al. [11, 12]. used a modified chemical-shift imaging (CSI) sequence with empirically-optimized pulse timing and a prior-knowledge based fitting to detect 2HG [11]. This sequence employs an echo time (TE) of 97 ms to detect resonances correspond to 2HG, but this comes with the limitation that quantification of other metabolites then becomes more difficult. A second strategy to detect 2HG is to use two-dimensional localized correlated spectroscopy (2D L-COSY) to introduce a second spectral dimension and identify 2HG by “cross-peak” resonances due to J-coupling interactions [13]. Using in vivo 2D L-COSY [14, 15], Ramadan et al. [16] have reported the detection of several metabolites such as PC, GPC, Lac and Glu/Gln from GBMs, indicating a potential utility of 2D L-COSY in further characterization of brain neoplasms. Though well-separated, these cross-peaks show lower signal intensity than primary resonances because they originate only from the small fraction of nuclei which undergo coherence transfer during t1-evolution, motivating a move to higher field strengths.
The introduction of ultrahigh field (7T) scanners improves the sensitivity of 1H MRS in the detection of 2HG and other complex metabolites due to greater signal to noise ratio and increased chemical shift dispersion. We have recently implemented 2D L-COSY at 7T [17], to reliably identify metabolites that were otherwise difficult to resolve with conventional 1H MRS, especially at lower field strength [11]. In this study, we aimed to test the efficacy of 2D L-COSY at 7T for detection of 2HG in IDH-mutated gliomas.
Methods
Subjects
The study was approved by the Institutional Review Board of the University of Pennsylvania and is compliant with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all patients prior to the study. A total of nine patients (five female, four male, ages 18–72 years, mean = 42 years) with suspected intracranial neoplasms based on clinical presentation and routine 3T MR imaging findings were recruited for this study. All 7T scans were performed following initial diagnosis and prior to surgical biopsy/tumor resection and chemo-radiation therapy. Histopathological and immunohistochemical analyses on the resected tumor specimens were subsequently performed and the findings were compared with the results from spectral data, specifically for tumor grade and IDH mutation status.
MR imaging and spectroscopy
All scans were performed on a Siemens (Siemens Healthcare, Erlangen, Germany) 7T MRI/MRS whole-body scanner equipped with a 32-channel transmit/receive head coil. Axial T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging was used to facilitate voxel localization on the intracranial neoplasms. Imaging parameters for the FLAIR sequence included: Echo time (TE) = 382 ms, repetition time (TR) = 4500 ms, TI = 2150 ms, 1 average, 240 × 240 mm2 field of view (FOV), 320x320 matrix size, 208 slices, 0.8 × 0.8 × 0.8 mm3 resolution, 6 min scan time.
A single voxel 2D L-COSY sequence was performed by selecting a voxel size of 8.8–18.0 ml from the solid portion of the neoplasm, carefully avoiding regions of hemorrhage/cysts. 2D L-COSY scan parameters were as follows: TE = 20 ms, TR = 2000 ms, 8 averages for each incremental period, 64 ∆t1 increments of 0.4 ms, 2048 t2 points with F2/F1 bandwidth = 4000/2500 Hz with a total scan time of 17 min. B1 field optimization was assisted by the automated FASTESTMAP (Siemens Healthcare) algorithm and water suppression was performed using the variable power and optimized relaxation delays (VAPOR) module [18].
Spectra from neoplasms were compared with data obtained from six healthy controls as part of a previous study [19]. In that study a voxel size of 27.0 ml encompassing bilateral cortical occipital region was used in all subjects. All other scan parameters for the control subjects were identical to those for the patients.
Data processing
Data from the 2D L-COSY studies were reconstructed offline using a custom MATLAB-based reconstruction program. Post-processing steps included Fourier transform, zero-filling, apodization using a squared shifted sine-bell windowing function and combining coil and averaging data. Metabolite signals were quantified through peak volume integration. The diagonal resonance of creatine (Cr) at [F2, F1] = 3.0, 3.0 ppm was used as an internal reference for computing metabolite ratios. Chemical shifts were calibrated using the resonance of NAA at [F2, F1] = 2.0, 2.0 ppm. Detection of 2HG was used to prospectively determine IDH mutation status and these findings were subsequently compared with the results from pathology.
Determination of IDH Status by Immunohistochemistry
Patient demographics along with histopathological grading and immuno-histochemical findings are presented in Table 1. Of the nine patients, one participant (patient #3) expressed physical discomfort during the scan and requested to terminate the study prematurely. 2D L-COSY data from two other subjects (patients #7 and #8, Table 1) had sub-optimal water suppression and produced spectra of insufficient quality for reliable metabolite quantification. Both of these spectra featured a broad resonance due to the residual signal from unsuppressed water in the region where the resonances due to 2HG, GPC, PC and Lac would be expected.
Tissue specimens received by pathology were fixed in formalin and processed for paraffin embedding. Hematoxylin and eosin staining and immunohistochemistry were conducted on 5-micron thick formalin-fixed, paraffin-embedded tissue sections mounted on Leica Surgipath slides followed by drying for 60 min at 70°. Immunohistochemistry with the anti-IDH1-R132H antibody (Monoclonal Mouse Anti-human IDH1 (R132H), Dianova, DIA Clone H09) and DAB chromogen was performed on a Leica Bond III instrument using Bond Polymer Refine Detection System (Leica Microsystems AR9800) following a 20-min heat-induced epitope retrieval with Epitope Retrieval 2, EDTA, pH 9.0.
Results
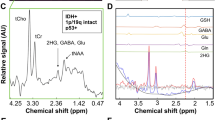
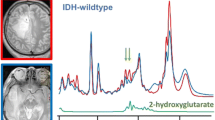
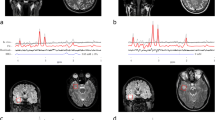
2D L-COSY spectra showed presence of 2HG at [F2, F1] = 4.0, 1.7 ppm in two of the six glioma cases (grade III anaplastic astrocytoma and grade II diffuse astrocytoma). None of wild-type IDH1 patients demonstrated the 2HG peak on the 2D L-COSY spectrum. IHC confirmed IDH1 mutation in both patients whose spectra showed presence of 2HG and IDH1 mutation was not detected in any of the other glial tumors studied. 2D L-COSY spectrum from a tumor harboring IDH1 mutation (patient #9) is shown in Fig. 1 with the voxel localization for the MRS shown on the T2-FLAIR image (inset). The diagonal peaks and cross-peak resonances due to coherence transfer in J-coupled metabolites are well-resolved and some of them have been labeled as: 2HG, Lac, lipids, GPC, PC, GPE + PE and mI + Cho. Representative 2D L-COSY spectrum from a patient with a wild-type IDH1 (patient #4) is shown in Fig. 2.
Besides NAA, Cr, tCho and mI that are typically resolved on 1D 1H MRS of brain neoplasms, resonances corresponding to other metabolites, e.g. PC, GPC, Glu, Gln, Lac and lipids were clearly resolved on 2D L-COSY spectra in each of the six patients. In addition, we also observed resonances corresponding amino acids such as aspartate (Asp) and lysine (Lys) not generally detected on 1D 1H MRS (Figs. 1, 2).
Figure 3 shows ratios of Lac and tCho with respect to creatine in each of the six brain tumor patients and normal controls. GPC/Cr and PC/Cr ratios were high in the only WHO grade III tumor studied, while these ratios were indistinguishable from normal controls in the patient with hematoma. An increasing trend in GPC, PC and total Cho was noticed with increasing tumor grade. No such trend was observed for the Lac signal.
Table 2 shows a list of ratios of cross-peak metabolite resonances with respect to Cr from the six patients and six normal subjects. These metabolites included Lac, lipids, lysine (Lys), Glu, Gln (and their combined resonance, Glx), NAA, Asp, mI, glycerophosphoethanolamine (GPE) and phosphoethanolamine (PE), the combined cross peaks of mI and free choline (mI + Cho), GPC and PC.
Discussion
To our knowledge, this is the first study of its kind in which 2D L-COSY has been used to characterize brain tumors at ultrahigh fields. The proportionately higher spectral separation on the 7T scanner improved specificity of detection of 2HG as well as other highly relevant metabolites in tumors, such as GPC, PC, Lac, Lys, Glu and Gln that are difficult to unambiguously resolve at lower fields.
Previous studies have reported that over 50 % of WHO Grade II/III gliomas harbor IDH mutations [1, 2]. In fact, the 2016 update to the WHO Classification of Tumors of the CNS has made this molecular change part of the diagnosis for the grade II/III “diffuse astrocytoma, IDH-mutant,” “anaplastic astrocytoma, IDH-mutant,” “oligodendroglioma, IDH-mutant and 1p/19q-codeleted” and “anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted.” Grade II “diffuse astrocytoma, IDH-wildtype” is a provisional entity given its rarity [8]. 2HG has been considered as a putative biomarker of these genotypes. A previous study [20] reported that patients with gliomas bearing IDH mutations show better response to chemo-radiation therapy and thus present favorable clinical outcome corroborating the need for 2HG detection to guide clinical management [20–23]. These mutations may also be candidates for targeted therapy (e.g. AGIOS 121) [24], making reliable detection of IDH mutation increasingly relevant to both the selection and monitoring of treatment efficacy, providing real-time feedback as a robust biomarker. Although IDH mutations can be identified by immunohistochemical analysis of resected tumor specimens [25–27], non-invasive detection of 2HG levels using 1H MRS techniques can be used to monitor targeted therapy. Detection of these biomarkers with 7T 2D L-COSY may inform the decision for change in therapy.
We observed 2HG in each of the two patients with gliomas bearing IDH mutation and in none of the four wild-type tumors, thus substantiating the usefulness of 7T 2D L-COSY in resolving the complex signals of 2HG overlapping with other metabolites. 1D 1H MRS studies have recently demonstrated [5, 11, 13, 28–31] in vivo detection of 2HG in patients with gliomas harboring IDH mutation. These published studies have typically been performed on clinical 3T scanners and relied on modified sequences with optimized echo-timing to resolve complex resonances of 2HG from Glu, Gln and GABA. This may limit the signal quality from other metabolites and thus confound their reliable quantification. Both of the 7T 1D MRS reporting 2HG utilized relatively long TEs (78 and 110 ms) for the purposes of signal optimization [12, 31], which may again limit assessment of short-T2 metabolites like Glu and Gln, particularly at such high field. It appears that 2D L-COSY represents an alternative technique for unambiguously detecting 2HG while simultaneously identifying additional clinically-relevant metabolites with high sensitivity.
In tumors, elevated total Cho levels, detected by 1H MRS, is indicative of increased cell membrane turnover and tumor malignancy [32, 33]. Moreover, relative amounts of PC and GPC have been suggestive of markers for predicting tumor grade [34] using in vitro 1H MRS. In an in vitro 1H MRS study of tumor extracts [34], it was reported that PC was the predominant total choline (tCho) peak in high grade gliomas, while GPC dominates in low grade gliomas and normal brain. The higher PC in high grade gliomas has been attributed to higher expression and/or activity of choline kinase, phospholipase C and Cho transporters that have been exploited as potential targets for therapy [35]. Furthermore, alterations in the PC/GPC ratio have been proposed as a marker of malignant transformation and treatment response [36]. Taken together, these studies provide adequate impetus to separate PC from GPC in vivo, to study tumor phospholipid metabolism, which can be studied using the 2D L-COSY technique as demonstrated in this study.
Detection of elevated Lac in neoplasms in our study is consistent with previous studies [37–39] and may reflect elevated tumor glycolysis and/or poor tissue perfusion. However, reliable detection of Lac on 1D 1H MRS is problematic due to presence of intense co-resonant lipids signals that are also elevated in brain neoplasms [40] and may also be present as an artifact from skull, as seen in patient #4 (Fig. 2). 2D L-COSY facilitates separation of Lac from background lipid signals through detection of the Lac cross-peak.
Brain neoplasms exploit Glx transporter expression and function to alter Glu-Gln homeostasis, which supports their growth, invasion, and survival [41]. Closely coupled to Glx, the role of GABA has also been studied in brain neoplasms [42, 43]. Consequently, reliable detection of these metabolites is valuable for studying tumor metabolism. In the present study, cross-peaks of Glu, Gln and GABA were clearly observed which may aid in better understanding of the tumor metabolism.
Spectral acquisition methods such as multiple quantum filtered spectroscopy and spectral editing techniques have been used to unravel potentially overlapping resonances [44]. However, technical restrictions associated with these sequences such as sensitivity to motion artifacts and constraint of observing only a single metabolite at a time render these techniques less attractive to study brain neoplasms in vivo [44]. On the other hand, 2D L-COSY provides improved signal, better dispersion of J-coupled peaks and detection of multiple metabolites in a single recording [15]. 2D L-COSY at 7T unambiguously resolved many overlapping resonances with improved signal and better chemical shift dispersion compared to 1D MRS or studies at lower field strengths [19] suggesting the increased sensitivity of ultra-high field 2D L-COSY in studying brain neoplasms as shown in this study.
This study was limited in sample size and by heterogeneity of tumor types primarily because the purpose was simply to demonstrate the feasibility of the sequence in assessing brain tumor metabolism and detecting 2HG. Follow-up studies are needed to focus on metabolic differences in specific tumor types. We acknowledge the limitations of our 2D-LCOSY technique with respect to 1D MRS in terms of relatively longer acquisition time. Future modifications of this sequence would benefit from acceleration techniques like matched accumulation [45] or sparse sampling to reduce overall scan time. Metabolite quantification of resonances detected in 2D L-COSY could also be improved by implementing a prior-knowledge based fitting approach analogous to LCModel [46] fitting on 1D MRS rather than the peak integration method that was used in the present study. ProFit [47] is one such 2D prior-knowledge based fitting algorithm, and adopting this program to 7T studies could improve the metabolite quantification.
Conclusion
We have demonstrated that 2D L-COSY has the ability to detect 2HG in vivo which has significant translational implications. We believe that 2D L-COSY may further improve our understanding of tumor physiology and metabolism and thus assist in better characterizing brain tumor genotypes in an era of molecular diagnostics and personalized medicine. As targeted therapies are developed for the IDH pathway, 2D-L-COSY could be used to detect 2HG as a key biomarker to evaluate treatment efficacy in a dynamic, iterative, real-time fashion, optimizing clinical trials and patient outcomes.
References
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73.
Ichimura K, Pearson DM, Kocialkowski S, Bäcklund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro-Oncology. 2009;11(4):341–7.
Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain tumor pathol. 2012;29(3):131–9.
Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP + -dependent isocitrate dehydrogenase mutations. PLoS ONE. 2011;6(2):e16812.
Pope WB, Prins RM, Thomas MA, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neuro-Oncol. 2012;107(1):197–205.
Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 2010;102(3):926–8.
Ducray F, Idbaih A, Wang X-W, Cheneau C, Labussiere M, Sanson M. Predictive and prognostic factors for gliomas. Expert Rev Anticancer Ther. 2011;11(5):781–9.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
Eliyahu G, Kreizman T, Degani H. Phosphocholine as a biomarker of breast cancer: molecular and biochemical studies. Int J Cancer. 2007;120(8):1721–30.
McKnight TR. Proton magnetic resonance spectroscopic evaluation of brain tumor metabolism. Semin Oncol. 2004;31:605–17.
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang X-L, Mashimo T, Raisanen JM, Marin-Valencia I. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–9.
Ganji SK, An Z, Tiwari V, McNeil S, Pinho MC, Pan E, Mickey BE, Maher EA, Choi C. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magn Reson Med. 2016. doi:10.1002/mrm.26190.
Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra114.
Thomas MA, Yue K, Binesh N, Davanzo P, Kumar A, Siegel B, Frye M, Curran J, Lufkin R, Martin P. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn Reson Med. 2001;46(1):58–67.
Thomas M, Hattori N, Umeda M, Sawada T, Naruse S. Evaluation of two-dimensional L-COSY and JPRESS using a 3 T MRI scanner: from phantoms to human brain in vivo. NMR Biomed. 2003;16:245–51.
Ramadan S, Andronesi OC, Stanwell P, Lin AP, Sorensen AG, Mountford CE. Use of in vivo two-dimensional MR spectroscopy to compare the biochemistry of the human brain to that of glioblastoma. Radiology. 2011;259(2):540–9.
Verma G, Hariharan H, Nagarajan R, Nanga RP, Delikatny EJ, Albert Thomas M, Poptani H. Implementation of two-dimensional L-COSY at 7 tesla: an investigation of reproducibility in human brain. J Magn Reson Imaging. 2014;40(6):1319–27.
Tkac I, Starcuk Z, Choi I, Gruetter R. In Vivo 1H NMR Spectroscopy of Rat Brain at 1 ms Echo Time. Magn Reson Med. 1999;41:649–56.
Verma G, Hariharan H, Nagarajan R, Nanga RP, Delikatny EJ, Albert Thomas M, Poptani H. Implementation of two-dimensional L-COSY at 7 tesla: an investigation of reproducibility in human brain. J Magn Reson Imaging. 2013;40:1319–27.
Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–6.
Dubbink H, Taal W, van Marion R, Kros J, van Heuvel I, Bromberg J, Zonnenberg B, Zonnenberg C, Postma T, Gijtenbeek J. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73(21):1792–5.
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4.
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–18.
Popovici-Muller J, Saunders JO, Salituro FG, Travins JM, Yan S, Zhao F, Gross S, Dang L, Yen KE, Yang H. Discovery of the first potent inhibitors of mutant IDH1 that lower tumor 2-HG in vivo. ACS Med Chem Lett. 2012;3(10):850–5.
Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509–11.
Takano S, Tian W, Matsuda M, Yamamoto T, Ishikawa E, Kaneko MK, Yamazaki K, Kato Y, Matsumura A. Detection of IDH1 mutation in human gliomas: comparison of immunohistochemistry and sequencing. Brain Tumor Pathol. 2011;28(2):115–23.
Takano S, Kato Y, Yamamoto T, Kaneko MK, Ishikawa E, Tsujimoto Y, Matsuda M, Nakai K, Yanagiya R, Morita S. Immunohistochemical detection of IDH1 mutation, p53, and internexin as prognostic factors of glial tumors. J Neuro-Oncol. 2012;108(3):361–73.
Kalinina J, Carroll A, Wang L, Yu Q, Mancheno DE, Wu S, Liu F, Ahn J, He M, Mao H. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med. 2012;90(10):1161–71.
Elkhaled A, Jalbert LE, Phillips JJ, Yoshihara HA, Parvataneni R, Srinivasan R, Bourne G, Berger MS, Chang SM, Cha S. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4(116):116ra115.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44.
Emir UE, Larkin SJ, de Pennington N, Voets N, Plaha P, Stacey R, Al-Qahtani K, Mccullagh J, Schofield CJ, Clare S. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer Res. 2016;76(1):43–9.
Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neuro-Oncol. 2000;50(3):215–26.
Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107(8–9):1027–63.
Sabatier J, Gilard V, Malet-Martino M, Ranjeva J, Terral C, Breil S, Delisle M, Manelfe C, Tremoulet M, Berry I. Characterization of choline compounds with in vitro 1H magnetic resonance spectroscopy for the discrimination of primary brain tumors. Investig Radiol. 1999;34(3):230–5.
Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem. 2003;90(3):525–33.
Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001;61(9):3599–603.
Sijens PE, Levendag PC, Vecht CJ, Dijk Pv, Oudkerk M. 1H MR spectroscopy detection of lipids and lactate in metastatic brain tumors. NMR Biomed. 1996;9(2):65–71.
Ott D, Hennig J, Ernst T. Human brain tumors: assessment with in vivo proton MR spectroscopy. Radiology. 1993;186(3):745–52.
Kugel H, Heindel W, Ernestus R, Bunke J, Du Mesnil R, Friedmann G. Human brain tumors: spectral patterns detected with localized H-1 MR spectroscopy. Radiology. 1992;183(3):701–9.
Howe F, Barton S, Cudlip S, Stubbs M, Saunders D, Murphy M, Wilkins P, Opstad K, Doyle V, McLean M. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49(2):223–32.
Shanware NP, Mullen AR, DeBerardinis RJ, Abraham RT. Glutamine: pleiotropic roles in tumor growth and stress resistance. J Mol Med. 2011;89(3):229–36.
Peeling J, Sutherland G. High-Resolution 1H NMR spectroscopy studies of extracts of human cerebral neoplasms. Magn Reson Med. 1992;24(1):123–36.
Nelp TB, McGovern RA, McKhann GM. Why glioma patients seize: adding more pathological GABA to the glutamate hypothesis. Neurosurgery. 2014;75(6):N10–1.
Muruganandham M, Koutcher JA, Pizzorno G, He Q. In vivo tumor lactate relaxation measurements by selective multiple-quantum-coherence (Sel-MQC) transfer. Magn Reson Med. 2004;52(4):902–6.
Kumar A, Brown SC, Donlan ME, Meier BU, Jeffs PW. Optimization of two-dimensional NMR by matched accumulation. J Magn Reson. 1991;95(1):1–9.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9.
Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19(2):255–63.
Authors’ contributions
Sequence Development: HP, NR, MT, GV, Scanning: SC, SM, HP, GV, SW, Post-processing & Quantification: NR, SC, MT, GV, SW, Pathology (Histopathology & Immunohistochemistry): MN. Radiology (Assessment of neoplasms): SM. Neurosurgery: SB, JL, Primary manuscript writing: GV, SM. All authors read and approved the final manuscript.
Acknowledgements
Tejiun Zhao of Siemens is acknowledged for providing the FASTESTMAP shim WIP package. The authors would like to acknowledge the scientific contributions of Dr. Ravi Prakash Reddy Nanga of the Center for Advanced Magnetic Resonance Imaging and Spectroscopy (CAMRIS). The authors would also like to thank the Hospital of the University of Pennsylvania (HUP) coordinators Lisa Desiderio, Katelyn O’Reilly and Matthew Voluck and MRI technicians, especially Jacqui Meeks, for their contributions to the project.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data is available from the corresponding authors upon reasonable request.
Consent for publication
Consent for publication of non-identifiable patient data is has been obtained through the institutional consent form approved by the IRB.
Ethics approval and consent to participate
All studies were performed with patient consent and with approval from the Institutional Review Board (IRB) of the University of Pennsylvania. All data was handled in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Funding
Funding for 7T studies was provided by a University of Pennsylvania Department of Radiology Protocol Development (ProDev) Grant. Dr. S. Brem receives research support, in part, from the NIH (R01 NS096606).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gaurav Verma and Suyash Mohan contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Verma, G., Mohan, S., Nasrallah, M.P. et al. Non-invasive detection of 2-hydroxyglutarate in IDH-mutated gliomas using two-dimensional localized correlation spectroscopy (2D L-COSY) at 7 Tesla. J Transl Med 14, 274 (2016). https://doi.org/10.1186/s12967-016-1035-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-016-1035-1