Abstract
Purpose
Combined radiochemotherapy (RCT) for gastric cancer with three-dimensional conformal radiotherapy (3D-CRT) results in ablative doses to the upper left kidney, while image-guided intensity-modulated radiotherapy (IG-IMRT) allows kidney sparing despite improved target coverage. Renal function in long-term gastric cancer survivors was evaluated with 3T functional magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI) and 23Na imaging.
Patients and methods
Five healthy volunteers and 13 patients after radiotherapy were included: 11×IG-IMRT; 1×3D-CRT; 1× “positive control” with stereotactic body radiotherapy (SBRT) of a metastasis between the spleen/left kidney. Radiation doses were documented for the upper/middle/lower kidney subvolumes. Late toxicity was evaluated based on CTC criteria, questionnaire, and creatinine values. Morphological sequences, DWI images, and 23Na images were acquired using a 1H/23Na-tuned body-coil before/after intravenous water load (WL). Statistics for [23Na] (concentration) and apparent diffusion coefficient (ADC) values were calculated for upper/middle/lower renal subvolumes. Corticomedullary [23Na] gradients and [23Na] differences after WL were determined.
Results
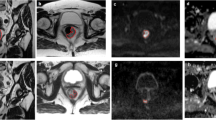
No major morphological alteration was detected in any patient. Minor scars were observed in the cranial subvolume of the left kidney of the 3D-CRT and the whole kidney of the control SBRT patient. All participants presented a corticomedullary [23Na] gradient. After WL, a significant physiological [23Na] gradient decrease (p < 0.001) was observed in all HV and IG-IMRT patients. In the cranial left kidney of the 3D-CRT patient and the positive control SBRT patient, the decrease was nonsignificant (p = 0.01, p = 0.02). ADC values were altered nonsignificantly in all renal subvolumes (all participants). Renal subvolumes with doses ≥ 35 Gy showed a reduced change of the [23Na] gradient after WL (p = 0.043). No participants showed clinical renal impairment.
Conclusions
Functional parameters of renal 23Na MRI after gastric IG-IMRT are identical to those of healthy volunteers, in contrast to renal subvolumes after ablative doses in the control and 3D-CRT patient. While kidney doses to the cortex below 20–25 Gy in fractional doses of ~ 1 Gy in IG-IMRT (combined with intensified chemotherapy) do not seem to cause significant MRI morphological or functional alterations, doses of > 35 Gy in 1.5–2 Gy fractions clearly result in impairment.
Zusammenfassung
Zielsetzung
Die Radiochemotherapie (RCT) des Magenkarzinoms mit 3-dimensionaler konformaler Strahlentherapie (3D-CRT) belastet den linken Nierenoberpol mit ablativen Strahlendosen. Die bildgeführte, intensitätsmodulierte Strahlentherapie (IG-IMRT) ermöglicht die Aussparung des Nierenparenchyms bei verbesserter Zielvolumenabdeckung. Die Nierenfunktion wurde mit 3 T-funktioneller MRT (diffusionsgewichtete Bildgebung; DWI) und 23Na-Bildgebung in einer Kohorte von Patienten mit langem Follow-up ausgewertet.
Patienten/Methoden
Es wurden 5 gesunde Probanden (HV) und 12 Patienten mit Magenkarzinom (11-mal IG-IMRT; 1-mal 3D-CRT) sowie ein Patient als „positive-Kontrolle“ (nach Behandlung einerMetastase zwischen Milz und linker Niere mit SBRT [„stereotactic-body-radiotherapy“]) untersucht. Die Bestrahlungsdosen wurden für die oberen, mittleren und unteren Nierenetagen getrennt dokumentiert. Spättoxizität wurde basierend auf den allgemeinen Toxizitätskriterien (CTC, Common Toxicity Criteria), mittels Fragebogen und anhand der Kreatininwerte bestimmt. Morphologische Sequenzen, DWI- und 23Na-Bilder wurden mit einer 1H/23Na-Spule vor und nach intravenöser Flüssigkeitszufuhr („water load“, WL) akquiriert. Die statistische Auswertung erfolgte für [23Na]-Konzentration und ADC („apparent diffusion coefficient“)-Werte getrennt nach Subvolumina (obere, mittlere und untere Nierenetagen). Kortikomedulläre [23Na]-Gradienten und [23Na]-Unterschiede nach WL wurden bestimmt.
Ergebnisse
Grobmorphologische Veränderungen wurden nicht detektiert. Kleine narbige Veränderungen wurden im linken Nierenoberpol (3D-CRT) und in der ganzen Niere des SBRT-Kontollpatienten beobachtet. Alle Untersuchten zeigten physiologische kortikomedulläre [23Na]-Gradienten. Nach WL wurde in allen HV und IG-IMRT-Patienten ein signifikanter physiologischer Abfall des [23Na]-Gradienten beobachtet (p < 0,001). Im linken Nierenoberpol des 3D-CRT-Patienten und dem „positivKontroll-SBRT“-Patienten war dieser Abfall nicht signifikant (p = 0,01; p = 0,02). Die ADC-Werte änderten sich in allen Subvolumina nicht signifikant. Subvolumina, die mit Dosen ≥ 35 Gy bestrahlt wurden, zeigten einen eingeschränkten Abfall des [23Na]-Gradienten nach WL (p = 0,043). Kein Patient wies eine klinische Symptomatik auf.
Schlussfolgerung
Funktionelle Parameter der 23Na-MRT der Niere nach adjuvanter IG-IMRT sind identisch zu gesunden Probanden, im Gegensatz zu Nierensubvolumina, die mit ablativen Dosen belastet wurden. Während kortikale Dosen unter 20–25 Gy in Fraktionsdosen von ~ 1 Gy nach IG-IMRT (kombiniert mit intensivierter Chemotherapie) keine MR-morphologischen oder funktionellen Veränderungen verursachen, resultieren Dosen von > 35 Gy in Fraktionsdosen von 1,5–2 Gy in manifesten Beeinträchtigungen.



Similar content being viewed by others
References
Boda-Heggemann J, Mennemeyer P, Wertz H et al (2009) Accuracy of ultrasound-based image guidance for daily positioning of the upper abdomen: an online comparison with cone beam CT. Int J Radiat Oncol Biol Phys 74:892–897
Boda-Heggemann J, Hofheinz RD, Weiss C et al (2009) Combined adjuvant radiochemotherapy with IMRT/XELOX improves outcome with low renal toxicity in gastric cancer. Int J Radiat Oncol Biol Phys 75:1187–1195
Boda-Heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M (2011) kV cone-beam CT-based IGRT: a clinical review. Strahlenther Onkol 187:284–291
Boda-Heggemann J, Guckenberger M, Ganswindt U et al (2012) Image-guided radiation therapy. Der Radiologe 52:213–221
Boda-Heggemann J, Weiss C, Schneider V et al (2013) Adjuvant IMRT/XELOX radiochemotherapy improves long-term overall- and disease-free survival in advanced gastric cancer. Strahlenther Onkol 189:417–423
Dikken JL, van Sandick JW, Maurits Swellengrebel HA et al (2011) Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 11:329
Fakhrian K, Ordu AD, Lordick F et al (2014) Long-term outcomes of trimodality treatment for squamous cell carcinoma of the esophagus with cisplatin and/or 5-FU: More than 20 years’ experience at a single institution. Strahlenther Onkol (epub ahead of print)
Guckenberger M, Meyer J, Wilbert J, Baier K, Sauer O Flentje M (2007) Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther Onkol 183:307–313
Haneder S, Konstandin S, Morelli JN et al (2011) Quantitative and qualitative 23Na MR imaging of the human kidneys at 3 T: before and after a water load. Radiology 260:857–865
Haneder S, Juras V, Michaely HJ et al (2013) In vivo sodium (Na) imaging of the human kidneys at 7 T: preliminary results. Eur Radiol 24(2):494–501
Haneder S, Kettnaker P, Konstandin S et al (2013) Quantitative in vivo (23)Na MR imaging of the healthy human kidney: determination of physiological ranges at 3.0 T with comparison to DWI and BOLD. Magma 26:501–509
Haneder S, Konstandin S, Morelli JN, Schad LR, Schoenberg SO, Michaely HJ (2013) Assessment of the renal corticomedullary (23)Na gradient using isotropic data sets. Acad Radiol 20:407–413
Haneder S, Michaely HJ, Schoenberg SO et al (2012) Assessment of renal function after conformal radiotherapy and intensity-modulated radiotherapy by functional 1 H-MRI and 23Na-MRI. Strahlenther Onkol 188:1146–1154
Haneder S, Michaely HJ, Konstandin S et al (2013) 3 T Renal Na-MRI: effects of desmopressin in patients with central diabetes insipidus. Magma 27(1):47–52.
Hofheinz RD, Wenz F, Lukan N et al (2009) Oxaliplatin and capecitabine-based chemoradiotherapy for gastric cancer-an extended phase I MARGIT and AIO trial. Int J Radiat Oncol Biol Phys 73:142–147
Huber K (2013) Cancer of the esophagus and gastroesophageal junction: improved survival with neoadjuvant radiochemotherapy. Strahlenther Onkol 189:161–162
Jansen EP, Boot H, Saunders MP et al (2007) A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 69:1424–1428
Jansen EP, Saunders MP, Boot H et al (2007) Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 67:781–785
Lee J, Lim do H, Kim S et al (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30:268–273
Li LP, Halter S, Prasad PV (2008) Blood oxygen level-dependent MR imaging of the kidneys. Magn Reson Imaging Clin N Am 16:613–625
Lohr F, Dobler B, Mai S et al (2003) Optimization of dose distributions for adjuvant locoregional radiotherapy of gastric cancer by IMRT. Strahlenther Onkol 179:557–563
Macdonald JS, Fleming TR, Peterson RF et al (1995) Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: a Southwest Oncology Group study. Ann Surg Oncol 2:488–494
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81:684–689
Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR (2009) Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med 62:1565–1573
Ohri N, Garg MK, Aparo S et al (2013) Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 86:330–335
Smalley SR, Benedetti JK, Haller DG et al (2012) Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 30:2327–2333
Thoeny HC, Zumstein D, Simon-Zoula S et al (2006) Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology 241:812–821
Welz S, Hehr T, Kollmannsberger C, Bokemeyer C, Belka C Budach W (2007) Renal toxicity of adjuvant chemoradiotherapy with cisplatin in gastric cancer. Int J Radiat Oncol Biol Phys 69:1429–1435
Wieland P, Dobler B, Mai S et al (2004) IMRT for postoperative treatment of gastric cancer: covering large target volumes in the upper abdomen: a comparison of a step-and-shoot and an arc therapy approach. Int J Radiat Oncol Biol Phys 59:1236–1244
Xu X, Fang W, Ling H, Chai W Chen K (2010) Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol 20:978–983
Acknowledgments
Parts of these studies were supported by research grants from Elekta AB, Stockholm, Sweden, and Siemens Healthcare, Erlangen, Germany.
J. Boda-Heggemann is supported by the Ministerium für Bildung und Forschung, Baden-Württemberg and the ESF (European Social Funds).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Haneder, J.M. Budjan, S.O. Schoenberg, S. Konstandin, L.R. Schad, R.D. Hofheinz, V. Gramlich, F. Wenz, F. Lohr and J. Boda-Heggemann state that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Haneder, S., Budjan, J., Schoenberg, S. et al. Dose-dependent changes in renal 1H-/23Na MRI after adjuvant radiochemotherapy for gastric cancer. Strahlenther Onkol 191, 356–364 (2015). https://doi.org/10.1007/s00066-014-0787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0787-x
Keywords
- Gastric cancer
- Functional 23Na-MRI
- Diffusion-weighted imaging
- Image-guided IMRT
- Kidney radiation tolerance