Abstract
Objectives
The purpose of this prospective study was to assess the normal physiologic ranges of the renal corticomedullary 23Na-concentration ([23Na]) gradient at 3.0T in healthy volunteers. The corticomedullary [23Na] gradient was correlated with other functional MR imaging parameters—blood oxygenation level dependent (BOLD) and diffusion-weighted imaging (DWI)—and to individual and physiologic parameters—age, gender, estimated glomerular filtration rate (eGFR), body mass index (BMI), and blood serum sodium concentration ([23Na]serum).
Methods and materials
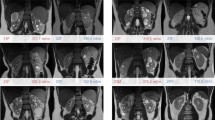
50 healthy volunteers (30 m, 20 w; mean age: 29.2 years) were included in this IRB-approved study, without a specific a priori preparation in regard to water or food intake. For 23Na-imaging a 3D density adapted, radial gradient echo (GRE)-sequence (spatial resolution = 5 × 5 × 5 mm3) was used in combination with a dedicated 23Na-coil and 23Na-reference phantoms. [23Na] values of the corticomedullary [23Na] gradient were measured by placement of a linear region of interest (20 × 1 mm2) from the renal cortex in the direction of the renal medulla. By using external standard reference phantoms, [23Na] was calculated in mmol/L of wet tissue volume (mmol/l WTV). Axial diffusion-weighted images (spatial resolution = 1.7 × 1.7 × 5.0 mm3) and 2D GRE BOLD images (spatial resolution = 1.2 × 1.2 × 4.0 mm3) were acquired. Mean values ± standard deviations for [23Na], apparent diffusion coefficient (ADC) values, and R2* values were computed for each volunteer. The corticomedullary 23Na-concentration gradient (in mmol/l/mm) was calculated along the area of linear concentration increase from the cortex in the direction of the medulla. Correlations between the [23Na] and DWI, BOLD, and the physiologic parameters were assessed with Pearson correlation coefficients.
Results
The mean corticomedullary [23Na] for all healthy volunteers increased from the renal cortex (58 ± 17 mmol/l WTV) in the direction of the medulla (99 ± 18 mmol/l WTV). The inter-individual differences ranged from respective cortical and medullary values of 27 and 63 mmol/L WTV to 126 and 187 mmol/L WTV. No statistically significant differences in renal [23Na] were found based on differences in individual or physiologic parameters (age, gender, [23Na]serum, BMI, GFR). No ADC or R2* gradients were identified, and [23Na] did not correlate with these parameters.
Conclusion
Renal corticomedullary [23Na] values increase from the cortex in the direction of the medullary pyramid, demonstrating wide inter-individual ranges and no significant correlations with age, gender, [23Na]serum, BMI, GFR, ADC, or R2* values. For future clinical evaluations, an approach relying on renal stimulation (e.g. pharmacologically induced diuresis) may be applicable to account for wide inter-individual ranges of normal [23Na].




Similar content being viewed by others
References
Michaely HJ, Herrmann KA, Nael K, Oesingmann N, Reiser MF, Schoenberg SO (2007) Functional renal imaging: nonvascular renal disease. Abdom Imaging 32(1):1–16
Michaely HJ, Kramer H, Attenberger U et al (2007) Renal magnetic resonance angiography at 3.0 T: technical feasibility and clinical perspectives. Top Magn Reson Imaging 18(2):117–125
Michaely HJ, Kramer H, Oesingmann N et al (2007) Intraindividual comparison of MR-renal perfusion imaging at 1.5 T and 3.0 T. Invest Radiol 42(6):406–411
Xu X, Fang W, Ling H, Chai W, Chen K (2010) Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol 20(4):978–983
Thoeny HC, Zumstein D, Simon-Zoula S et al (2006) Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology 241(3):812–821
Li LP, Halter S, Prasad PV (2008) Blood oxygen level-dependent MR imaging of the kidneys. Magn Reson Imaging Clin N Am 16(4):613–625
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI (2012) Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81(7):684–689
Haneder S, Konstandin S, Morelli JN et al (2011) Quantitative and qualitative 23Na MR Imaging of the Human Kidneys at 3 T: before and after a water load. Radiology 260(3):857–865
Maril N, Rosen Y, Reynolds GH, Ivanishev A, Ngo L, Lenkinski RE (2006) Sodium MRI of the human kidney at 3 Tesla. Magn Reson Med 56(6):1229–1234
Maril N, Margalit R, Rosen S, Heyman SN, Degani H (2006) Detection of evolving acute tubular necrosis with renal 23Na MRI: studies in rats. Kidney Int 69(4):765–768
Maril N, Margalit R, Mispelter J, Degani H (2004) Functional sodium magnetic resonance imaging of the intact rat kidney. Kidney Int 65(3):927–935
Rosen Y, Lenkinski RE (2009) Sodium MRI of a human transplanted kidney. Acad Radiol 16(7):886–889
Haneder S, Michaely HJ, Schoenberg SO, Konstandin S, Schad LR, Siebenlist K, Wertz H, Wenz F, Lohr F, Boda-Heggemann J (2012) Assessment of renal function after conformal radiotherapy and intensity modulated radiotherapy by functional 1H-MRI and 23Na-MRI. Strahlenther Onkol 188(12):1146–1154
Lanz T, Mayer M, Robson MD, Neubauer S, Ruff J, Weisser A (2007) An 8 Channel 23Na Heart Array for Application at 3T. In: Proceedings of the 15th scientific meeting, international society for magnetic resonance in medicine, Berlin, p 241
Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR (2009) Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med 62(6):1565–1573
Konstandin S, Nagel AM (2012) Performance of sampling density-weighted and post filtered density-adapted projection reconstruction in sodium magnetic resonance imaging. Magn Reson Med. doi:10.1002/mrm.24255
Furlan A, Moon C-H, Kim J-H, He X, Park B, Zaho T, Bae KT (2011) Sodium MR imaging of human kidney using a dual-tuned (23Na/1H) body RF coil at 3T: quantitative assessment of sodium concentration and cortico medullary gradient in healthy subjects. In: Proceedings of the 19th scientific meeting, International Society for Magnetic Resonance in Medicine, Montreal, p 2947
Prabhakar SS (2004) Regulatory and functional interaction of vasoactive factors in the kidney and extracellular pH. Kidney Int 66(5):1742–1754
Kohan DE, Rossi NF, Inscho EW, Pollock DM (2011) Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91(1):1–77
Holley JL (2004) The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis 11(4):337–341
Notohamiprodjo M, Reiser MF, Sourbron SP (2010) Diffusion and perfusion of the kidney. Eur J Radiol 76(3):337–347
Toya R, Naganawa S, Kawai H, Ikeda M (2010) Correlation between estimated glomerular filtration rate (eGFR) and apparent diffusion coefficient (ADC) values of the kidneys. Magn Reson Med Sci 9(2):59–64
Acknowledgments
We thank Dr. Armin Nagel for providing the modified, density-adapted 23Na pulse sequence. Parts of these studies were supported by a research grant from Deutsche Forschungsgemeinschaft (DFG) SCHO 710/2-2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haneder, S., Kettnaker, P., Konstandin, S. et al. Quantitative in vivo 23Na MR imaging of the healthy human kidney: determination of physiological ranges at 3.0T with comparison to DWI and BOLD. Magn Reson Mater Phy 26, 501–509 (2013). https://doi.org/10.1007/s10334-013-0369-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-013-0369-4