Abstract
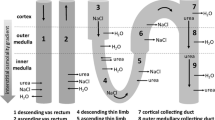
Modelling studies have played an important role in research on the mechanism of urine concentration and dilution by the medulla of the kidney ever since Hargitay and Kuhn (1951,Z. Elektrochem. 55, 539–558) first proposed that the parallel tubular structures in the kidney medulla must function as a “countercurrent multiplication” system. Present-day models, in keeping with our considerably improved understanding of most aspects of medullary structure-function relationships, have evolved into rather sophisticated systems of parallel tubes. In spite of this increasing complexity, it has remained the case that “model medullas” do not concentrate as well as the real kidney, especially in the inner medulla where only passive, diffusional transport occurs. Inasmuch as these models take into account the majority of contemporary ideas making up our global hypothesis about the functioning of this system, their failure to behave physiologically indicates that our understanding remains incomplete. The purpose of the present modelling study was to evaluate the implications of some recent measurements showing that permeabilities of NaCl (P s ) and urea (P u ) vary along the length of the descending thin limbs of Henle (Imaiet al., 1988,Am. J. Physiol. 254, F323–F328), rather than being constant throughout this segment as had been assumed earlier. It was hoped that these newly measured values might explain, by a passive, diffusional process, the net solute addition at the bend of Henle’s loop observed under some circumstances and heretofore attributed (though without any supporting experimental evidence) to active transport into the descending limb. The results of the present study show that whereas incorporation of the new values forP s andP u in the descending limbs of short nephrons does indeed improve the concentrating power of the model, these new values are nonetheless not sufficient to allow the model to build an osmolarity gradient that increases all the way through the inner medulla. This failing, which is common to virtually all modelling studies to date using measured values from rat kidneys, probably points to a key role for preferential exchange supposed by some to exist among certain tubule segments within vascular bundles in species whose kidneys have the highest concentrating power.
Similar content being viewed by others
Abbreviations
- OM:
-
outer medulla
- IM:
-
inner medulla
- DHL:
-
descending Henle’s limbs (generic term for all SDL and LDL)
- SDL:
-
short descending loop of Henle
- LDL:
-
long descending loop of Henle
- ATL:
-
ascending thin limb
- TAL:
-
thick ascending limb
- SDT:
-
distal tubule of short nephrons
- LDT:
-
distal tubule of long nephrons
- CD:
-
collecting duct (tube 4)
- DVR:
-
descending vasa recta (tube 5)
- AVR:
-
ascending vasa recta (tube 6), also assumes the role of Interstitium
- i :
-
tube number (i=1, ..., 21)
- j :
-
solute number (j=1 for salt,j=2 for urea)
- x :
-
distance along the cortico-medullary axis (mm)
- F iv :
-
axial volume flow along tubei (mm3 s−1)
- F ij :
-
axial flow of solutej along tubei (nmol s−1), =F iv ·c ij
- J iv :
-
transmural volume flux out of tubei per unit tube length (mm2 sec−1)
- J ij :
-
transmural solute flux out of tubei per unit tube length (nmol mm−1sec−1)
- c ij :
-
concentration of solutej in tubei (mmol l−1=nmol mm−3)
- Lp i :
-
hydraulic permeability of tubei (mm sec−1 (mosm/l)−1)
- P ij :
-
permeability of tubei to solutej (mm sec−1)
- N i :
-
number of tubesi at a given depth,x, in the medulla
- r i :
-
radius of tubei (mm)
- A i :
-
total circumference of tubesi(=2πr i N i ) (mm)
- σ ij :
-
reflection coefficient of tubei to solutej
- a ij :
-
maximum rate of active transport (nmol mm−2 sec−1)
- b ij :
-
Michaelis constant (mmol l−1)
- RT :
-
universal gas constant times temperature (0.02545 atm (mmol/l)−1 at 37°C)
Literature
Andreoli, T. E., R. W. Berliner, J. P. Kokko and D. J. Marsh. 1978. Questions and replies: renal mechanisms for urinary concentrating and diluting processes.Am. J. Physiol. 235 (Renal 4-1), F1-F11.
Foster, D. M. and J. A. Jacquez. 1978. Comparison using central core model of renal medulla of the rabbit and rat.Am. J. Physiol. 234 (Renal 3), F402-F414.
Gottschalk, C. W. 1961. Micropuncture studies of tubular function in the mammalian kidney.Physiologist 4, 35–55.
Hargitay, B. and W. Kuhn. 1951. Das Multiplikationsprinzip als Grundlage der Harnkonzentrierung in der Niere.Z. Elektrochem 55, 539–558.
Imai, M., J. Taniguchi and K. Yoshitomi. 1988. Transition of permeability properties along the descending limb of long loops nephron.Am. J. Physiol. 254 (Renal 23), F323-F328.
Jamison, R. L. and W. Kriz. 1982.Urinary Concentrating Mechanism: Structure and Function. New York: Oxford University Press.
Jen, J. F. and J. L. Stephenson. 1990. Equations for externally driven central core multiplier.FASEB J. 4(3), A686 (Abstract No. 2435).
Kainer, R. 1975. A geometric model of the kidney.Anat. Embryol. 147, 91–109.
Kaissling, B. and W. Kriz. 1979.Structural Analysis of Rabbit Kidney. Berlin: Springer.
Knepper, M. A. 1982. Measurement of osmolality in kidney slices using vapor pressure osmometry.Kidney Int. 21, 653–658.
Knepper, M. A. and M. Burg. 1983. Organization of nephron function.Am. J. Physiol. 244 (Renal 13), F579-F589.
Kokko, J. P. and F. Rector. 1972. Countercurrent multiplication system without active transport in inner medulla.Kidney Int. 2, 214–223.
Kriz, W. 1981. Structural organization of the renal medulla: comparative and functional aspects.Am. J. Physiol. 241, Rs-R16.
Lassiter, W. E., C. W. Gottschalk and M. Mylle. 1961. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney.Am. J. Physiol. 200, 1139–1146.
Layton, H. E. 1990. Urea transport in a distributed loop model of the urine-concentrating mechanism.Am. J. Physiol. 58, F1110-F1124.
Lory, P. 1987. Effectiveness of a salt transport cascade in the renal medulla: computer simulations.Am. J. Physiol. 252, F1095-F1102.
Lory, P., A. Gilg and M. Horster. 1983. Renal countercurrent system: role of collecting duct convergence of pelvic urea predicted from a mathematical model.J. math. Biol. 16, 281–304.
Marsh, D. J. and L. Segel. 1971. Analysis of countercurrent exchange in blood vessels of the renal medulla.Am. J. Physiol. 221 (Renal 3), F1095-F1102.
Mejia, R. and J. L. Stephenson. 1979. Numerical solution of multinephron kidney equations.J. Comput. Phys. 32, 235–246.
Mejia, R., J. M. Sands, J. L. Stephenson and M. A. Knepper. 1989. Renal actions of atrial natriuretic factor: a mathematical modeling study.Am. J. Physiol. 257 (Renal 26), F1146-F1157.
Moore, L. C. and D. J. Marsh. 1980. How descending limb of Henle’s loop permeability affects hypertonic urine formation.Am. J. Physiol. 239 (Renal 8), F57-F71.
Pennel, J. P., F. B. Lacy and R. L. Jamison. 1975. Anin vivo study of the concentrating process in the descending lumb of Henle’s loop.Kidney Int. 5, 337–347.
Press, W. H., B. P. Flannery, S. A. Teukolsky and W. T. Vetterling. 1986.Numerical Methods. The Art of Scientific Computing. Cambridge.
Stephenson, J. L. 1972. Concentration of urine in a central core model of the renal counter flow system.Kidney Int. 2, 85–94.
Stephenson, J. L. 1976. Concentrating engines and the kidney. III. Canonical mass balance equation for multinephron models of the renal medulla.Biophys. J. 16, 1273–1286.
Stephenson, J. L. and J. F. Jen 1990. Limiting concentration ratios for two modes of operation of a central core model of the renal medulla.FASEB J. 4(3): A686, (Abstract No. 2436).
Stephenson, J. L., R. P. Tewarson and R. Mejia. 1974. Quantitative analysis of mass and energy balance in non-ideal models of the renal counterflow system.Proc. natn. Acad. Sci. U.S.A. 71, 1618–1622.
Taniguchi, J., K. Tabei and M. Imai. 1987. Profiles of water and solute transport along long-loop descending limb: analysis by mathematical model.Am. J. Physiol. 252 (Renal 21), F393-F402.
Tewarson, R. P., J. L. Stephenson, M. Garcia and Y. Zhang. 1985. On the solution of equations for renal counterflow models.Comp. Bol. Med. 15(5), 287–295.
Thomas, S. R. and D. C. Mikulecky. 1978. Transcapillary solute exchange. A comparison of the Kedem-Katchalsky convection-diffusion equations with the rigorous nonlinear equations for this special case.Microvascular Res. 15, 207–220.
Wexler, A. S., R. E. Kalaba and D. J. Marsh. 1986. Automatic derivative evaluation in solving boundary value problems: the renal medulla.Am. J. Physiol. 251 (Renal 20), F358-F378.
Wexler, A. S., R. E. Kalaba and D. J. Marsh. 1987. Passive, one-dimensional countercurrent models do not simulate hypertonic urine formation.Am. J. Physiol. 253 (Renal 22), F1020-F1030.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomas, S.R. Effect of varying salt and urea permeabilities along descending limbs of Henle in a model of the renal medullary urine concentrating mechanism. Bltn Mathcal Biology 53, 825–843 (1991). https://doi.org/10.1007/BF02461486
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02461486