Abstract
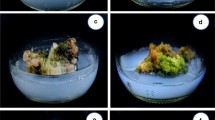
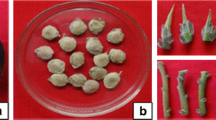
Nothapodytes nimmoniana Graham is an important medicinal tree species occurring in the Western Ghats, a mega diversity hotspot in Southern India. The inner stem bark of the tree contains an important anti-cancer alkaloid, camptothecine (CTP), for which the natural population of the trees is heavily extracted. In this paper, we report the development of a rapid, high frequency regeneration protocol from leaf and nodal explants of N. nimmoniana. Multiple shoot induction was carried out using leaf and nodal explants on Murashige and Skoog (MS) medium supplemented with different concentration/combination of phyto-hormones. N6-benzyladenine (BA) was the most effective cytokinin for the induction of multiple shoots. The MS medium with 8.87 µM BA yielded the highest number of shoots from leaf and nodal explants, respectively. Further, proliferation and elongation of adventitious buds were observed in secondary medium containing MS supplemented with 4.44 µM BA and 0.87 µM gibberellic acid (GA3). Shoots were rooted on half strength MS medium containing 4.9 µM indole-3-butyric acid. The plantlets were acclimatized in a growth chamber at 25 °C, 60 % relative humidity, with 16/8 h light/dark photoperiod. Regenerated plants were free of any noticeable phenotypic variability and showed a survival rate of 90 %. The in vitro regenerated plants accumulated substantial amount of camptothecine (ranging from 0.08 to 0.2 %). These results suggest the possibility of using in vitro regenerated plants as a possible alternative source of CPT. This is the first report of direct regeneration in N. nimmoniana with significantly high plant regeneration frequency and with high CPT yield.



Similar content being viewed by others
References
Ciddi, V., & Shuler, M. L. (2000). Camptothecine from callus culture of Nothapodytes foetida. Biotechnology Letters, 22, 129–132.
Dandin, V. S., & Murthy, H. N. (2012). Enhanced in vitro multiplication of Nothapodytes nimmoniana Graham using semisolid and liquid cultures and estimation of camptothecine in the regenerated plants. Acta Physiologiae Plantarum, 34, 1381–1386. doi:10.1007/s11738-012-0934.
Emmanuel, S., Ignacimuthu, S., & Kathiravan, K. (2000). Micropropagation of Wedelia calendulacea Less., a medicinal plant. Phytomorphology, 50(2), 195–200.
Gomez, K. A., & Gomez, A. A. (1976). Statistical procedures for agricultural research with emphasis on rice. Los Banos: International Rice Research Institute.
Govindachari, T. R., & Viswanathan, N. (1972). Alkaloids of Mappia foetida. Phytochemistry, 11, 3529–3531.
Hombe Gowda, H. C., Vasudeva, R., Georgi, P. M., Uma, S. R., & Ganeshaiah, K. N. (2002). Breeding types in Nothapodytes nimmoniana Graham. Current Science, 83, 1077–1078.
Hsiang, Y. H., Hertzberg, R., Hecht, S., & Liu, L. F. (1985). Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase-I. Journal of Biological Chemistry, 260(27), 14873–14878.
Kai, G., Dai, L., Mei, X., Zheng, J., Wang, W., Qian, Z., & Zhou, G. (2008). In vitro plant regeneration from leaf explants of Ophiorrhiza japonica. Biologia Plantarum, 52(3), 557–560.
Kathiravan, K., & Ignacimuthu, S. (1999). Micropropagation of Canavalia virosa (ROXB) Wight & Arn. A medicinal plant. Phytomorphology, 49, 61–66.
Kawiak, A., Krolicka, A., & Lojkowska, E. (2003). Direct regeneration of Drosera from leaf explants and shoot tips. Plant Cell, Tissue and Organ Culture, 75, 175–178.
Lattoo, S. K., Bamotra, S., SapruDhar, R., & Khan, S. (2006). Rapid plant regeneration and analysis of genetic fidelity of in vitro derived plants of Chlorophytum arundinaceum Baker—An endangered medicinal herb. Plant Cell Reports, 25, 499–506. doi:10.1007/s00299-005-0103-4.
Lorence, A., & Craig L. N. (2004) Camptothecin: over four decades of surprising findings. Phytochemistry, 65(20), 2731–2841.
Lorence, A., Medina, B. F., & Nessler, C. L. (2004). Camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata hairy roots. Plant Cell Reports, 22, 437–441.
Martin, K. P., Sunandakumari, C., Chithra, M., & Madhusoodanan, P. V. (2005). Influence of auxins in direct in vitro morphogenesis of Euphorbia nivulia, a lectinacious medicinal plant. In Vitro Cellular & Developmental Biology-Plant, 41(3), 314–319.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Namdeo, A. G., Priya, T., & Bhosale, B. B. (2012). Micropropagation and production of camptothecine from in vitro plants of Ophiorrhiza mungos. Asian Pacific Journal of Tropical Biomedicine, 2(2 supplement), S662–S666.
Neeti, D., & Kothari, S. L. (2005). Micropropagation of Eclipta alba (L.) Hassk—An important medicinal plant. In Vitro Cellular & Developmental Biology-Plant, 41, 658–6661.
Padmanabha, B. V., Chandrashekar, M., Ramesha, B. T., Hombe, G. H. C., Gunaga, R. P., Suhas, S., et al. (2006). Pattern of accumulation of Camptothecin, an anticancer alkaloid in Nothapodytes nimmoniana Graham. In the Western Ghats, India: Implications for identifying high yielding sources of alkaloid. Current Science, 90(1), 95–99.
Priel, E., Showalter, S. D., & Blair, D. G. (1991). Inhibition of human immunodeficiency virus (HIV-I) replication in vitro by noncytotoxic doses of camptothecine, a topoisomerase I inhibitor. AIDS Research and Human Retroviruses, 7, 65–72.
Purohit, D., & Dave, A. (1996). Micropropagation of Sterculia aurens Roxb.—An endangered tree species. Plant Cell Reports, 15, 704–706.
Rai, R. V. (2002). Rapid clonal propagation of Nothapodytes foetida (Wight) Sleumer—A threatened medicinal tree. In Vitro Cellular & Developmental Biology-Plant, 38, 347–351.
Ramadevi, T., Ugraiah, A., & Pullaiah, T. (2012). In vitro shoot multiplication from nodal explants of Boucerosia diffusa Wight—An endemic medicinal plant. Indian Journal of Biotechnology, 11, 344–347.
Ramesha, B. T., Amna, T., Ravikanth, G., Rajesh, P. G., Vasudeva, R., Ganeshaiah, K. N., et al. (2008). Prospecting for camptothecine from Nothapodytes nimmoniana in the Western Ghats, South India: Identification of yielding sources of camptothecine and new families of camptothecines. Journal of Chromatographic Science, 46, 362–368.
Ramesha, B. T., Suma, H. K., Senthilkumar, U., Priti, V., Ravikanth, G., Vasudeva, R., et al. (2013). New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine, 20, 521–527.
Ravi kumar, K., & Ved, D. K. (2000). 100 Red listed medicinal plants of conservation concern in Southern India (pp. 261–263). Bangalore: FRLHT.
Roja, M., & Heble, M. R. (1994). The quinoline alkaloids camptothecine and 9-methoxycamptothecine from tissue culture and mature trees of Nothapodytes foetida. Phytochemistry, 36, 65–66.
Rout, G. R. (2005). Direct plant regeneration of Curry leaf tree (Murraya koenigii Koenig.), an aromatic plant. In Vitro Cellular & Developmental Biology-Plant, 41(2), 133–136.
Sajeevan, R. S., Singh, S. J., Nataraja, K. N., & Shivanna, M. B. (2011). An efficient in vitro protocol for multiple shoot induction in mulberry, Morus alba L variety V1. International Research Journal of Plant Sciences, 2(8), 254–261.
Shahanaz, B. A., Martin, K. P., Zhang, Chun-Lai, Nishitha, I. K., Ligimol, Slater A., & Madhusoodanan, P. V. (2007). Organogenesis from leaf and internode explants of Ophiorrhiza prostrata, an anticancer drug (Camptothecine) producing plant. Electronic Journal of Biotechnology, 10(1), 1–10.
Tavares, A. C., Salgueiro, L. R., & Canhoto, J. M. (2010). In vitro propagation of the wild carrot Daucus carota L. subsp. halophilus (Brot.) A. Pujadas for conservation purpose. In Vitro Cellular & Developmental Biology-Plant, 46, 47–56.
Thejavathi, D. H., Raveesha, H. R., & Shobha, K. (2011). Evaluation of different treatments to improve the seed germination among the populations of Nothapodytes foetida (WT.) Sleumer. Indian Journal of Fundamental and Applied Life Sciences, 4, 187–192.
Thengane, S. R., Kulkarni, D. K., Shrikhande, V. A., Joshi, S. P., Sonawane, K. B., & Krishnamurthy, K. V. (2003). Influence of medium composition on callus induction and camptothecine accumulation in Nothapodytes foedita. Plant Cell, Tissue and Organ Culture, 72, 247–251.
Thengane, S. R., Kulkarni, D. K., Shrikhande, V. A., & Krishnamurthy, K. V. (2001). Effect of thidiazuron on adventitious shoot regeneration from seedling explants of Nothapodytes foetida. In Vitro Cellular & Developmental Biology-Plant, 37, 206–210.
Thomas, T. D., & Shankar, S. (2009). Multiple shoot induction and callus regeneration in Sarcostemma brevistigma Wight & Arnott, a rare medicinal plant. Plant Biotechnology Reports, 3, 67–74.
Ugraiah, A., Raja Sreelatha, V., Krishna Reddy, P. V., Rajasekhar, K., Sandhya Rani, S., Karuppusamy, S., & Pullaiah, T. (2011). In vitro shoot multiplication and conservation of Caralluma bhupenderiana Sarkaria—An endangered medicinal plant from South India. African Journal of Biotechnology, 10(46), 9328–9336.
Ulukan, H., & Swaan, P. W. (2002). Camptothecine: A review of their chemotherapeutic potential. Drugs, 62(14), 2039–2057.
Uma, S. R., Ramesha, B. T., Ravikanth, G., Gunaga, R., Vasudeva, R., & Ganeshaiah, K. N. (2008). Chemical profiling of Nothapodytes nimmoniana for camptothecine, an important anticancer alkaloid: Toward the development of a sustainable production system. In K. G. Ramawat & J. M. Merillon (Eds.), Bioactive molecules and medicinal plants (pp. 197–213). UK: Springer Publishing.
Verma, S., Magotra, R., & Koul, A. K. (2002). In vitro multiplication of Eremurus persicus Boiss. (Liliaceae)—An endangered species. Phytomorphology, 52, 315–321.
Wall, M. E., Wani, M. C., Cook, C. E., Palmer, K. H., McPhail, A. T., & Sim, G. A. (1966). Plant antitumor agents. I. The isolation and structure of camptothecine, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. Journal of the American Chemical Society, 88, 3888–3890.
Wang, H. M., Zu, Y. G., Wang, W. J., Wu, S. X., & Dong, F. L. (2006). Establishment of Camptotheca acuminate regeneration from leaf explants. Biologia Plantarum, 50, 725–728.
Yamazaki, Y., Sudo, H., Yamazaki, M., Aimi, N., & Saito, K. (2003). Camptothecine biosynthetic genes in hairy roots of Ophiorrhiza pumila: Cloning, characterization and differential expression in tissues and by stress compounds. Plant and Cell Physiology, 44, 395–403.
Zhang, L., Kai, G., Xu, T., Pi, Y., Zhang, H., Sun, X., & Tang, K. (2004). Efficient regeneration of tetraploid Isatis indigotica plants via adventitious organogenesis from hypocotyls explants. Biologia Plantarum, 48, 121–124.
Acknowledgments
Authors are thankful to Science and Engineering Research Board (SERB), Department of Science and Technology (file no. SB/YS/LS-151/2013), New Delhi for its funding assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amilineni, U., Gangal, V., Gudasalamani, R. et al. Establishment and standardization of in vitro regeneration protocol in Nothapodytes nimmoniana Graham and evaluation of camptothecine (CPT) in tissue culture plants . Ind J Plant Physiol. 21, 1–7 (2016). https://doi.org/10.1007/s40502-015-0182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0182-3