Abstract
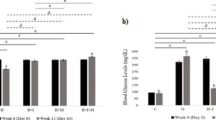
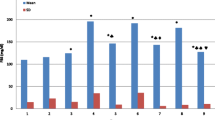
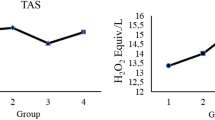
Oxidative stress-mediated damage to liver tissue underlies the pathological alterations in liver morphology and function that are observed in diabetes. We examined the effects of the antioxidant action of melatonin against necrosis-inducing DNA damage in hepatocytes of streptozotocin (STZ)-induced diabetic rats. Daily administration of melatonin (0.2 mg/kg) was initiated 3 days before diabetes induction and maintained for 4 weeks. Melatonin-treated diabetic rats exhibited improved markers of liver injury (P < 0.05), alkaline phosphatase, and alanine and aspartate aminotransferases. Melatonin prevented the diabetes-related morphological deterioration of hepatocytes, DNA damage (P < 0.05), and hepatocellular necrosis. The improvement was due to containment of the pronecrotic oxygen radical load, observed as inhibition (P < 0.05) of the diabetes-induced rise in lipid peroxidation and hydrogen peroxide increase in the liver. This was accompanied by improved necrotic markers of cellular damage: a significant reduction in cleavage of the DNA repair enzyme poly(ADP-ribose) polymerase 1 (PARP-1) into necrotic 55- and 62-kDa fragments, and inhibition of nucleus-to-cytoplasm translocation and accumulation in the serum of the high-mobility group box 1 (HMGB1) protein. We conclude that melatonin is hepatoprotective in diabetes. It reduces extensive DNA damage and resulting necrotic processes. Melatonin application could thus present a viable therapeutic option in the management of diabetes-induced liver injury.




Similar content being viewed by others
References
Anwar MM, Meki ARM (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp Biochem Physiol A 135:539–547
Arab JP, Ramírez C, Arrese M (2010) Early warning of liver disease in diabetics. Ann Hepatol 9:307–309
Arambasić J, Mihailović M, Bogojević D, Ivanović Matić S, Uskoković A, Poznanović G, Grigorov I (2013) Haptoglobin and the inflammatory and oxidative status in experimental diabetic rats: antioxidant role of haptoglobin. J Physiol Biochem 69:45–58
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Bell DS, Allbright E (2007) The multifaceted associations of hepatobiliary disease and diabetes. Endocr Pract 13:300–312
Bell CW, Jiang W, Reich CF III, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 29:C1318–C1325
Bouchard VJ, Rouleau M, Poirier GG (2003) PARP-1, a determinant of cell survival in response to DNA damage. A review. Exp Hematol 31:446–454
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, Pozo D (2006) The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs 7:423–431
Chatila R, West AB (1996) Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine 75:327–333
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
D’Amours D, Sallmann FR, Dixit VM, Poirier GG (2001) Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 114:3771–3778
Ditsworth D, Zong WX, Thompson CB (2007) Activation of poly(ADP)-ribose polymerase (PARP1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 282:17845–17854
Erlandsson HH, Andersson U (2004) Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 34:1503–1512
Filipović DM, Meng X, Reeves WB (1999) Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol 277:F428–F436
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Gobeil S, Bouscher CC, Nadeau D, Poirier GG (2001) Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 8:588–594
Harrison SA, Brunt EM, Goodman ZD, Di Bisceglie AM (2006) Diabetic hepatosclerosis: diabetic microangiopathy of the liver. Arch Pathol Lab Med 130:27–32
Hoijman E, Viegas LR, Sarmiento MIK, Rosenstein RE, Pecci A (2004) Involvement of Bax protein in the prevention of glucocorticoid-induced thymocytes apoptosis by melatonin. Endocrinology 145:418–425
Kim MY, Zhang T, Kraus WL (2005) Poly(ADP-ribosyl)ation by PARP-1: ‵PAR-laying′ NAD+ into a nuclear signal. Genes Dev 19:1951–1967
Lobenhofer EK, Boorman GA, Phillips KL, Heinloth AN, Malarkey DE, Blackshear PE, Houle C, Hurban P (2006) Application of visualization tools to the analysis of histopathological data enhances biological insight and interpretation. Toxicol Pathol 34:921–928
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342
Maritim AC, Moore BH, Sanders RA, Watkins JB III (1999) Effects of melatonin on oxidative stress in streptozotocin-induced diabetic rats. Int J Toxicol 18:161–166
Mathes AM (2010) Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. A review. World J Gastroenterol 16:6087–6097
Montilla PL, Vargas JF, Túnez IF, Muñoz de Agueda MC, Valdelvira ME, Cabrera ES (1998) Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J Pineal Res 25:94–100
Nishida S (2005) Metabolic effects of melatonin on oxidative stress and diabetes mellitus. Endocrine 27:131–136
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pang SF, Tang F, Tang PL (1985) Alloxan induced diabetes and the pineal gland: differential effects on the levels of pineal N-acetylserotonin, pineal melatonin, and serum melatonin. J Pineal Res 2:79–85
Park KS, Kim JH, Kim MS, Kim JM, Kim SK, Choi JY, Chung MH, Han B, Kim SY, Lee HK (2001) Effects of insulin and antioxidant in plasma 8-hydroxyguanine and tissue 8-hydroxydeoxyguanosine in streptozotocin-induced diabetic rats. Diabetes 50:2837–2841
Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38:161–170
Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59:365–373
Raucci A, Palumbo R, Bianchi ME (2007) HMGB1: a signal of necrosis. Autoimmunity 40:285–289
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. A review J Biomed Sci 7:444–458
Rubartelli A, Lotze MT (2007) Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28:429–436
Sailaja Devi MM, Suresh Y, Das UN (2000) Preservation of the antioxidant status in chemically-induced diabetes mellitus by melatonin. J Pineal Res 29:108–115
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Shah GM, Shah RG, Poirier GG (1996) Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem Biophys Res Commun 229:838–844
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Soldani C, Scovassi AI (2002) Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 7:321–328
Stebelová K, Herichová I, Zeman M (2007) Diabetes induces changes in melatonin concentrations in peripheral tissues of rat. Neuro Endocrinol Lett 28:159–165
Tang D, Kang R, Zeh HJ III, Lotze MT (2011) High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14:1315–1335
Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X (2007) Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 81:741–747
Tentori L, Turriziani M, Franco D, Serafino A, Levati L, Roy R, Bonmassar E, Graziani G (1999) Treatment with temozolomide and poly(ADP-ribose) polymerase inhibitors induces early apoptosis and increases base excision repair gene transcripts in leukemic cells resistant to triazene compounds. Leukemia 13:901–909
Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier G, Salvesen GS, Dixit VM (1995) Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81:801–809
Tolman KG, Fonseca V, Dalpiaz A, Tan MH (2007) Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30:734–743
Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204:2913–2923
Tutuncu NB, Batur MK, Yildirir A, Tutuncu T, Deger A, Koray Z, Erbas B, Kabakci G, Aksoyek S, Erbas T (2005) Melatonin levels decrease in type 2 diabetic patients with cardiac autonomic neuropathy. J Pineal Res 39:43–49
Viràg L, Scott GS, Cuzzocrea S, Marmer D, Salzman AL, Szabó C (1998) Peroxynitrite-induced thymocyte apoptosis: the role of caspases and poly (ADP-Ribose) synthetase (PARS) activation. Immunology 94:345–355
Viràg L, Szabó C (2002) The therapeutic potential of poly(ADP-Ribose) polymerase inhibitors. Pharmacol Rev 54:375–429
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant no. 173020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grigorov, I., Bogojević, D., Jovanović, S. et al. Hepatoprotective effects of melatonin against pronecrotic cellular events in streptozotocin-induced diabetic rats. J Physiol Biochem 70, 441–450 (2014). https://doi.org/10.1007/s13105-014-0322-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-014-0322-7