Abstract
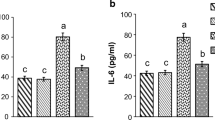
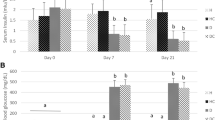
Haptoglobin is a hemoglobin-binding acute-phase protein which possesses anti-inflammatory and antioxidative properties. In this study, we investigated changes in protein expression of rat haptoglobin under diabetes-related inflammatory and oxidative stress conditions induced by an i.p. injection of streptozotocin. The progress of diabetes during an 8-week follow-up period was associated with the increased presence of haptoglobin in the serum and in the liver. This increase was most prominent during the first 2 weeks after which it started to decline. Temporary changes in haptoglobin expression strongly correlated with the serum levels of TNF-α and IL-6. Lower haptoglobin expression at the fourth week and thereafter correlated with a decrease in TNF-α concentration and changes in the TNF-α/IL-6 ratio. Based on the decrease of GSH/GSSG ratio and antioxidant enzyme activities in the liver until the end of fourth week, it was concluded that the liver was exposed to oxidative stress and injury which in the presence of the abovementioned inflammatory mediators lead to different haptoglobin expression profiles at different stages of diabetes. An inverse correlation was observed between the haptoglobin and free iron serum levels in diabetic rats. The higher levels of haptoglobin during the first 2 weeks were accompanied by a lower level of free iron. In view of the established function of haptoglobin, we discuss its possible role in decreasing oxidative stress during the early stage of diabetes.





Similar content being viewed by others
References
Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP (2005) Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res 96:435–441
Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP (2003) Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res 92:1193–1200
Auclair C, Voisin E (1985) Nitroblue tetrazolium reduction. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC, Boca Raton, pp 123–132
Baumann H, Prowse KR, Marinković S, Won K-A, Jahreis GP (1989) Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci 557:280–295
Beutler E (1982) Catalase. In: Beutler E (ed) Red cell metabolism: a manual of biochemical methods. Grune and Stratton, New York, pp 105–106
Courtoy PJ, Lombart C, Feldmann G, Moguilevsky N, Rogier E (1981) Synchronous increase of four acute phase proteins synthesized by the same hepatocytes during the inflammatory reaction: a combined biochemical and morphologic kinetics study in the rat. Lab Invest 44:105–115
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383
Cussimanio BL, Booth AA, Todd P, Hudson BG, Khalifah RG (2003) Unusual susceptibility of heme proteins to damage by glucose during non-enzymatic glycation. Biophys Chem 105:743–755
Delanghe JR, Langlois MR, Boelaert JR, van Acker J, van Wanzeele F, van der Groen G, Hemmer R, Verhofstede C, De Buyzere M, De Bacquer D, Arendt V, Plum J (1998) Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS 12:1027–1032
Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK (2006) Serum IL-1β, IL-2 and IL-6 in insulin-dependent diabetic children. Mediators Inflamm 2006:1–6
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglicemia in humans. Role of oxidative stress. Circulation 106:2067–2072
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 340:448–454
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamdena K, Carreaub S, Elfeki A (2009) Inhibitory effects of Zinc on hyperglycaemia and metabolic disorders in the liver of alloxan-induced diabetic rats. Asian Biomed 3:745–750
Hanley JM, Haugen TH, Health EC (1983) Biosynthesis and processing of rat haptoglobin. J Biol Chem 258:7858–7869
Herrman CE, Sanders RA, Klaunig JE, Schwarz LR, Watkins JB (1999) Decreased apoptosis as a mechanism for hepatomegaly in streptozotocin-induced diabetic rats. Toxicol Sci 50:146–151
Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J (1998) Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. J Clin Sci 94:623–632
Kruger JA, Yang C, Tam WS, Hinerfeld D, Evans EJ, Green MK, Leszyk J, Yang K, Guberski LD, Mordes PJ, Greiner LD, Rossini AA, Bortell R (2010) Haptoglobin as an early serum biomarker of virus-induced autoimmune type 1 diabetes in biobreeding diabetes resistant and LEW1.WR1 rats. Exp Biol Med 235:1328–1337
Laight DW, Carrier MJ, Anggard EE (1999) Endothelial cell dysfunction and the pathogenesis of diabetic macroangiopathy. Diabetes Metab Res Rev 15:274–282
Leclercq IA, Morais ADS, Schroyen B, Hul NV, Geerts A (2007) Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepat 47:142–156
Lenk SE, Bhat D, Blakeney W, Dunn WA Jr (1992) Effects of streptozotocin-induced diabetes on rough endoplasmic reticulum and lysosomes of rat liver. Am J Physiol 263:856–862
Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H (1998) Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92:1870–1877
Loven D, Schedl H, Wilson H, Diekus M (1986) Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes 35:503–507
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
McMillan DE (1989) Increased levels of acute-phase serum proteins in diabetes. Metabolism 38:1042–1046
Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, Levy AP (2001) Structure-function analysis of the antioxidant properties of haptoglobin. Blood 98:3693–3698
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moreira LRS, Miranda-Virela AL, Silva ICR, Akimoto AK, Klautau-Guimarӓes MN, Grisolia CK (2009) Antioxidant effect of haptoglobin phenotypes against DNA damage induced by hydrogen peroxide in human leukocytes. Gen Mol Res 8:284–290
Nepomnyashchikh GI, Pavlenko OA, Aidagulova SV, Nepomnyashchikh DL (2001) Ultrastructural analysis of liver biopsy specimens in diabetes mellitus associated with chronic opisthorchiasis. Bull Exp Biol Med 132:1190–1194
Noorafshan A, Esmail-Zadeh B, Bahmanpour S, Poost-Pasand A (2005) Early stereological changes in liver of Sprague–Dawley rats after streptozotocin injection. Indian J Gastroenterol 24:104–107
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38:161–170
Pickup JC, Chusney GD, Thomas SM, Burt D (2000) Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 67:291–300
Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59:365–373
Rekna N, Balaji R, Deecaraman M (2008) Effect of aqueous extract of Syzygium cumini pulp on antioxidant defense system in streptozotocin induced diabetic rats. IJPT 7:137–145
Rossi R, Dalle-Donne I, Milzani A, Giustarini D (2006) Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem 52:1406–1414
Roy M, Sen S, Chakraborti AS (2008) Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci 82:1102–1110
Swaminathan S, Fonseca VA, Alam MG, Shah SV (2007) The role of iron in diabetes and its complications. Diabetes Care 30:1926–1933
Vadas P, Grouix B, Stefanski E, Wloch M, Pruzanski W, Schroeder J, Gauldie J (1997) Coordinate expression of group II phospholipase A2 and the acute-phase protein haptoglobin (HP) and α1-anti-chymotrypsin (ACH) by HepG2 cells. Clin Exp Immunol 108:175–180
Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 49:642–652
Acknowledgments
This work was supported by the Serbian Ministry of Education and Science, grant no. 173020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jelena, A., Mirjana, M., Desanka, B. et al. Haptoglobin and the inflammatory and oxidative status in experimental diabetic rats: antioxidant role of haptoglobin. J Physiol Biochem 69, 45–58 (2013). https://doi.org/10.1007/s13105-012-0186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0186-7