Abstract
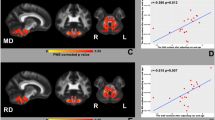
Friedreich’s ataxia (FRDA) is the commonest autosomal recessive ataxia, caused by GAA triplet expansion in the frataxin gene. Neuropathological studies in FRDA demonstrate that besides the primary neurodegeneration of the dorsal root ganglia, there is a progressive atrophy of the cerebellar dentate nucleus. Diffusion-weighted imaging (DWI) detected microstructural alterations in the cerebellum of FRDA patients. To investigate the biochemical basis of these alterations, we used both DWI and proton MR spectroscopy (1H-MRS) to study the same cerebellar volume of interest (VOI) including the dentate nucleus. DWI and 1H-MRS study of the left cerebellar hemisphere was performed in 28 genetically proven FRDA patients and 35 healthy controls. In FRDA mean diffusivity (MD) values were calculated for the same 1H-MRS VOI. Clinical severity was evaluated using the International Cooperative Ataxia Rating Scale (ICARS). FRDA patients showed a significant reduction of N-acetyl-aspartate (NAA), a neuroaxonal marker, and choline (Cho), a membrane marker, both expressed relatively to creatine (Cr), and increased MD values. In FRDA patients NAA/Cr negatively correlated with MD values (r = −0.396, p = 0.037) and with ICARS score (r = −0.669, p < 0.001). Age-normalized NAA/Cr loss correlated with the GAA expansion (r = −0.492, p = 0.008). The reduced cerebellar NAA/Cr in FRDA suggests that neuroaxonal loss is related to the microstructural changes determining higher MD values. The correlation between NAA/Cr and the severity of disability suggests that this biochemical in vivo MR parameter might be a useful biomarker to evaluate therapeutic interventions.


Similar content being viewed by others
References
Harding AE. Clinical features and classification of inherited ataxias. Adv Neurol. 1993;61:1–14. Review.
Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256 Suppl 1:3–8.
Marmolino D. Friedreich’s ataxia: past, present and future. Brain Res Rev. 2011;67(1-2):311–30.
Campuzano V, Montermini L, Moltò MD, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271(5254):1423–7.
Martelli A, Puccio H. Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front Pharmacol. 2014;5:130.
Martelli A, Napierala M, Puccio H. Understanding the genetic and molecular pathogenesis of Friedreich’s ataxia through animal and cellular models. Dis Model Mech. 2012;5(2):165–76.
Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet. 2000;9(2):275–82.
Calabrese V, Lodi R, Tonon C, et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci. 2005;233(1-2):145–62. Review.
Koeppen AH, Michael SC, Knutson MD, et al. The dentate nucleus in Friedreich’s ataxia: the role of iron-responsive proteins. Acta Neuropathol. 2007;114(2):163–73.
Lodi R, Tonon C, Calabrese V, Schapira AH. Friedreich’s ataxia: from disease mechanisms to therapeutic interventions. Antioxid Redox Signal. 2006;8(3-4):438–43. Review.
Lodi R, Cooper JM, Bradley JL, et al. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci U S A. 1999;96(20):11492–5.
Lodi R, Taylor DJ, Schapira AH. Mitochondrial dysfunction in friedreich’s ataxia. Biol Signals Recept. 2001;10:263–70.
Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 2011;122(3):323–30.
Koeppen AH, Mazurkiewicz JE. Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol. 2013;72(2):78–90.
Nilsson M, van Westen D, Ståhlberg F, Sundgren PC, Lätt J. The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter. MAGMA. 2013;26(4):345–70.
Rizzo G, Tonon C, Valentino ML, et al. Brain diffusion-weighted imaging in Friedreich’s ataxia. Mov Disord. 2011;26(4):705–12.
Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich’s ataxia. Neuroradiology. 2011;53(5):367–72.
Moats RA, Watson L, Shonk T, et al. Added value of automated clinical proton MR spectroscopy of the brain. J Comput Assist Tomogr. 1995;19(3):480–91.
Mascalchi M, Cosottini M, Lolli F, et al. Proton MR spectroscopy of the cerebellum and pons in patients with degenerative ataxia. Radiology. 2002;223(2):371–8.
Iltis I, Hutter D, Bushara KO, et al. (1)H MR spectroscopy in Friedreich’s ataxia and ataxia with oculomotor apraxia type 2. Brain Res. 2010;1358:200–10.
Fortuna F, Barboni P, Liguori R, et al. Visual system involvement in patients with Friedreich’s ataxia. Brain. 2009;132(Pt 1):116–23.
Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome, The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145(2):205–11.
Lodi R, Parchi P, Tonon C, et al. Magnetic resonance diagnostic markers in clinically sporadic prion disease: a combined brain magnetic resonance imaging and spectroscopy study. Brain. 2009;132(Pt 10):2669–79.
Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9.
Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–4.
Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335(16):1169–75.
Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington’s disease: correlations with CAG repeat numbers. Neurology. 1998;50(5):1357–65.
Waldvogel D, van Gelderen P, Hallett M. Increased iron in the dentate nucleus of patients with Friedrich’s ataxia. Ann Neurol. 1999;46(1):123–5.
Bonilha da Silva C, Bergo FPG, D’Abreu a, Cendes F, Lopes-Cendes I, França MC. Dentate nuclei T2 relaxometry is a reliable neuroimaging marker in Friedreich’s ataxia. Eur J Neurol. 2014;21(8):1131–6.
Solbach K, Kraff O, Minnerop M, et al. Cerebellar pathology in Friedreich’s ataxia: atrophied dentate nuclei with normal iron content. Neuroimage Clin. 2014;6:93–9.
Della Nave R, Ginestroni A, Tessa C, et al. Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel-based morphometry. NeuroImage. 2008;40(1):19–25.
Della Nave R, Ginestroni A, Giannelli M, et al. Brain structural damage in Friedreich’s ataxia. J Neurol Neurosurg Psychiatry. 2008;79(1):82–5.
França Jr MC, D’Abreu A, Yasuda CL, et al. A combined voxel-based morphometry and 1H-MRS study in patients with Friedreich’s ataxia. J Neurol. 2009;256(7):1114–20.
Vieira Karuta SC, Raskin S, de Carvalho NA, Gasparetto EL, Doring T, Teive HAG. Diffusion tensor imaging and tract-based spatial statistics analysis in Friedreich’s ataxia patients. Parkinsonism Relat Disord. 2015;21(5):504–8.
Akhlaghi H, Corben L, Georgiou-Karistianis N, et al. Superior cerebellar peduncle atrophy in Friedreich’s ataxia correlates with disease symptoms. Cerebellum. 2011;10(1):81–7.
Clemm von Hohenberg C, Schocke MF, Wigand MC, et al. Radial diffusivity in the cerebellar peduncles correlates with clinical severity in Friedreich ataxia. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2013;34(8):1459–62.
Viau M, Marchand L, Bard C, Boulanger Y. (1)H magnetic resonance spectroscopy of autosomal ataxias. Brain Res. 2005;1049(2):191–202.
McLean MA, Woermann FG, Barker GJ, Duncan JS. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magn Reson Med. 2000;44(3):401–11.
Ribaï P, Pousset F, Tanguy ML, et al. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol. 2007;64(4):558–64.
Tonon C, Lodi R. Idebenone in Friedreich’s ataxia. Expert Opin Pharmacother. 2008;9(13):2327–37.
Evans-Galea MV, Pébay A, Dottori M, et al. Cell and gene therapy for Friedreich ataxia: progress to date. Hum Gene Ther. 2014;25(8):684–493.
Acknowledgments
We thank Dr. Simonetta Righi (Biblioteca Centralizzata Clinica—University of Bologna) for her support in bibliographic research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The S. Orsola-Malpighi Hospital Ethics Committee approved the study, and the written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gramegna, L.L., Tonon, C., Manners, D.N. et al. Combined Cerebellar Proton MR Spectroscopy and DWI Study of Patients with Friedreich’s Ataxia. Cerebellum 16, 82–88 (2017). https://doi.org/10.1007/s12311-016-0767-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0767-z