Abstract
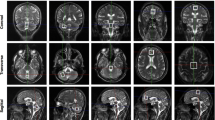
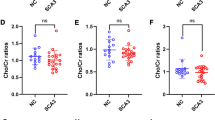
Autosomal dominant ataxia type 14 (SCA14) is a rare usually adult-onset progressive disorder with cerebellar neurodegeneration caused by mutations in protein kinase C gamma. We set out to examine cerebellar and extracerebellar neurochemical changes in SCA14 by MR spectroscopy. In 13 SCA14 patients and 13 healthy sex- and age-matched controls, 3-T single-voxel brain proton MR spectroscopy was performed in a cerebellar voxel of interest (VOI) at TE = 30 ms to obtain a neurochemical profile of metabolites with short relaxation times. In the cerebellum and in additional VOIs in the prefrontal cortex, motor cortex, and somatosensory cortex, a second measurement was performed at TE = 144 ms to mainly extract the total N-acetyl-aspartate (tNAA) signal besides the signals for total creatine (tCr) and total choline (tCho). The cerebellar neurochemical profile revealed a decrease in glutathione (6.12E−06 ± 2.50E−06 versus 8.91E−06 ± 3.03E−06; p = 0028) and tNAA (3.78E−05 ± 5.67E−06 versus 4.25E−05 ± 5.15E−06; p = 0023) and a trend for reduced glutamate (2.63E−05 ± 6.48E−06 versus 3.15E−05 ± 7.61E−06; p = 0062) in SCA14 compared to controls. In the tNAA-focused measurement, cerebellar tNAA (296.6 ± 42.6 versus 351.7 ± 16.5; p = 0004) and tCr (272.1 ± 25.2 versus 303.2 ± 31.4; p = 0004) were reduced, while the prefrontal, somatosensory and motor cortex remained unaffected compared to controls. Neuronal pathology in SCA14 detected by MR spectroscopy was restricted to the cerebellum and did not comprise cortical regions. In the cerebellum, we found in addition to signs of neurodegeneration a glutathione reduction, which has been associated with cellular damage by oxidative stress in other neurodegenerative diseases such as Parkinson’s disease and Friedreich’s ataxia.



Similar content being viewed by others
Abbreviations
- CHESS:
-
Chemical shift selective suppression
- tCho:
-
Total choline
- tCr:
-
Total creatine
- Glu:
-
Glutamate
- Gln:
-
Glutamine
- GSH:
-
Glutathione
- Glx:
-
The combined value of glutamate and glutamine
- HC:
-
Healthy controls
- Ins:
-
Inositol
- Lac:
-
Lactate
- tNAA:
-
N-Acetylaspartate
- NAAG:
-
NAA-Glutamate
- PRESS:
-
Point-resolved spectroscopy sequence
- SCA14:
-
Spinocerebellar ataxia type 14
- VOI:
-
Volume of interest
References
Chen D-H, Brkanac Z, Verlinde CLMJ et al (2003) Missense mutations in the regulatory domain of pkc: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet 72:839–849
Seki T, Adachi N, Ono Y et al (2005) Mutant protein kinase Cgamma found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death. J Biol Chem 280:29096–29106. doi:10.1074/jbc.M501716200
Shuvaev AN, Horiuchi H, Seki T et al (2011) Mutant PKCγ in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J Neurosci 31:14324–14334. doi:10.1523/JNEUROSCI.5530-10.2011
Chen D-H, Cimino PJ, Ranum LPW et al (2005) The clinical and genetic spectrum of spinocerebellar ataxia 14. Neurology 64:1258–1260. doi:10.1212/01.WNL.0000156801.64549.6B
van de Warrenburg BPC, Verbeek DS, Piersma SJ et al (2003) Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurology 61:1760–1765. doi:10.1212/01.WNL.0000098883.79421.73
Vlak MHM, Sinke RJ, Rabelink GM et al (2006) Novel PRKCG/SCA14 mutation in a Dutch spinocerebellar ataxia family: expanding the phenotype. Mov Disord 21:1025–1028. doi:10.1002/mds.20851
Wieczorek S, Arning L, Gizewski ER et al (2007) Benign SCA14 phenotype in a German patient associated with a missense mutation in exon 3 of the PRKCG gene. Mov Disord 22:2135–2136. doi:10.1002/mds.21673
Koht J, Stevanin G, Durr A et al (2012) SCA14 in Norway, two families with autosomal dominant cerebellar ataxia and a novel mutation in the PRKCG gene. Acta Neurol Scand 125:116–122. doi:10.1111/j.1600-0404.2011.01504.x
Wedding IM, Koht J, Dietrichs E et al (2013) Cognition is only minimally impaired in Spinocerebellar ataxia type 14 (SCA14): a neuropsychological study of ten Norwegian subjects compared to intrafamilial controls and population norm. BMC Neurol 13:186. doi:10.1186/1471-2377-13-186
Klebe S, Durr A, Rentschler A et al (2005) New mutations in protein kinase Cgamma associated with spinocerebellar ataxia type 14. Ann Neurol 58:720–729. doi:10.1002/ana.20628
Hiramoto K, Kawakami H, Inoue K et al (2006) Identification of a new family of spinocerebellar ataxia type 14 in the Japanese spinocerebellar ataxia population by the screening of PRKCG exon 4. Mov Disord 21:1355–1360. doi:10.1002/mds.20970
Oz G, Vollmers ML, Nelson CD et al (2011) In vivo monitoring of recovery from neurodegeneration in conditional transgenic SCA1 mice. Exp Neurol 232:290–298. doi:10.1016/j.expneurol.2011.09.021
Lopes TM, D’Abreu A, França MC Jr et al (2013) Widespread neuronal damage and cognitive dysfunction in spinocerebellar ataxia type 3. J Neurol 260:2370–2379. doi:10.1007/s00415-013-6998-8
Lirng J-F, Wang P-S, Chen H-C et al (2012) Differences between spinocerebellar ataxias and multiple system atrophy-cerebellar type on proton magnetic resonance spectroscopy. PLoS One 7:e47925. doi:10.1371/journal.pone.0047925
Hadjivassiliou M, Wallis LI, Hoggard N et al (2012) MR spectroscopy and atrophy in Gluten, Friedreich’s and SCA6 ataxias. Acta Neurol Scand 126:138–143. doi:10.1111/j.1600-0404.2011.01620.x
Boesch SM, Schocke M, Bürk K et al (2001) Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6. J Magn Reson Imaging 13:553–559
Boesch SM, Wolf C, Seppi K et al (2007) Differentiation of SCA2 from MSA-C using proton magnetic resonance spectroscopic imaging. J Magn Reson Imaging 25:564–569. doi:10.1002/jmri.20846
Doss S, Brandt AU, Oberwahrenbrock T et al (2014) Metabolic Evidence for Cerebral Neurodegeneration in Spinocerebellar Ataxia Type 1. Cerebellum 13:199–206. doi:10.1007/s12311-013-0527-2
Oz G, Hutter D, Tkác I et al (2010) Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord 25:1253–1261. doi:10.1002/mds.23067
Oz G, Iltis I, Hutter D et al (2011) Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum 10:208–217. doi:10.1007/s12311-010-0213-6
Kalbe E, Kessler J, Calabrese P et al (2004) DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry 19:136–143. doi:10.1002/gps.1042
Schmitz-Hübsch T, du Montcel ST, Baliko L et al (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720. doi:10.1212/01.wnl.0000219042.60538.92
Jacobi H, Rakowicz M, Rola R et al (2013) Inventory of Non-Ataxia Signs (INAS): validation of a new clinical assessment instrument. Cerebellum 12:418–428. doi:10.1007/s12311-012-0421-3
van de Warrenburg BPC, Verbeek DS, Piersma SJ et al (2003) Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurology 61:1760–1765. doi:10.1212/01.WNL.0000098883.79421.73
Brkanac Z, Bylenok L, Fernandez M et al (2002) A new dominant spinocerebellar ataxia linked to chromosome 19q13.4-qter. Arch Neurol 59:1291–1295. doi:10.1001/archneur.59.8.1291
Yamashita I, Sasaki H, Yabe I et al (2000) A novel locus for dominant cerebellar ataxia (SCA14) maps to a 10.2-cM interval flanked by D19S206 and D19S605 on chromosome 19q13.4-qter. Ann Neurol 48:156–163
Tyson RL, Sutherland GR (1998) Labeling of N-acetylaspartate and N-acetylaspartylglutamate in rat neocortex, hippocampus and cerebellum from [1-13C]glucose. Neurosci Lett 251:181–184
Neale JH, Bzdega T, Wroblewska B (2000) N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 75:443–452
Groger A, Kolb R, Schafer R, Klose U (2014) Dopamine reduction in the substantia nigra of parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS One. doi:10.1371/journal.pone.0084081
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4:1399–1440. doi:10.3390/nu4101399
Ristoff E, Larsson A (2007) Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis 2:16. doi:10.1186/1750-1172-2-16
Iltis I, Hutter D, Bushara KO et al (2010) (1)H MR spectroscopy in Friedreich’s ataxia and ataxia with oculomotor apraxia type 2. Brain Res 1358:200–210. doi:10.1016/j.brainres.2010.08.030
Lin D, Barnett M, Lobell S et al (2006) PKCgamma knockout mouse lenses are more susceptible to oxidative stress damage. J Exp Biol 209:4371–4378. doi:10.1242/jeb.02524
Holmay MJ, Terpstra M, Coles LD et al (2013) N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol 36:103–106. doi:10.1097/WNF.0b013e31829ae713
Acknowledgments
We thank Susan Pikol and Cynthia Kraut for excellent technical support and Hanna Zimmermann, Ella Kadas, Timm Oberwahrenbrock, and Leonora Zange for supporting the study logistics.
Conflicts of interest
SD received financial support for travel and research projects from Teva and Actelion and speakers honoraria from Actelion, SD reports no specific conflict of interest. JLR reports no conflict of interest. TSH received travel grants from Allergan. AUB is cofounder and associate of Motognosis. He received consulting fees, speaker honoraria and research grants from Novartis, Biogen Idec, Teva Pharmaceuticals, Bayer, and Heidelberg Engineering. He reports no conflict of interest in regard to the current study. SP and SM report no conflict of interest. JW serves on advisory boards for Novartis and Biogen Idec. He received a research grant from Novartis, and speaker honoraria from Bayer, Novartis, Teva and Biogen Idec. He is supported by the German ministry of science (BMBF/KKNMS). ME has no direct conflict of interests concerning this manuscript. He has received grant support from AstraZeneca and Sanofi, has participated in advisory board meetings of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Pfizer, Sanofi and has received honoraria from Astra Zeneca, Bayer, Berlin Chemie, Bristol-Myers Squibb, Boehringer-Ingelheim, Desitin, Eisei, Ever, Glaxo Smith Kline, MSD, Novartis, Pfizer, Sanofi, Takeda, Trommsdorff. TK receives/has received research support from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF) and the European Union (EU). He has received a lecture honorarium from Lundbeck and from Biogen Idec. He receives/has received royalties for book publications from Thieme, Urban & Schwarzenberg, Kohlhammer, Elsevier, Wissenschaftliche Verlagsgesellschaft Stuttgart and M. Dekker. MM reports no conflict of interest. FP reports to have received grant support from Sanofi, Beyer, Pfizer, and Teva where he sees no direct conflict of interest with this work. FP is supported by the Deutsche Forschungsgemeinschaft (DFG Exc 257), the Bundesministerium für Bildung und Forschung (BMBF Competence Network Multiple Sclerosis), and the European Union (FP 7, combims.eu).
Ethical standard
The study was approved by the local ethics committee of the Charité - Universitätsmedizin Berlin and the University of Bonn and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave informed written consent.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Doss, J. L. Rinnenthal, M. Minnerop, and F. Paul contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doss, S., Rinnenthal, J.L., Schmitz-Hübsch, T. et al. Cerebellar neurochemical alterations in spinocerebellar ataxia type 14 appear to include glutathione deficiency. J Neurol 262, 1927–1935 (2015). https://doi.org/10.1007/s00415-015-7788-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7788-2