Abstract
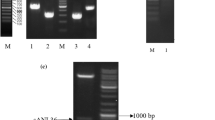
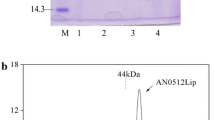
A 1,080-bp cDNA (CGMCC 2873) encoding of a cold-active lipase of Aspergillus fumigatus (AFL67) was cloned and expressed in Escherichia coli for the first time. The new lipase, AFL67, was one-step purified by 8.30 folds through Ni–NTA affinity chromatography with a recovery of 86.8 %. The specific activity of purified AFL67 was 449 U mg−1 on p-NP hexanoate. AFL67 preferentially hydrolyzed p-nitrophenyl esters of short- and medium-chain fatty acids, with p-nitrophenyl hexanoate the maximum. The optimum temperature and pH was 15 °C and 7.5, respectively. The purified AFL67 was stable at 10–25 °C for 30 min, and in the pH range of 6.0–9.0 for 16 h (at 4 °C). Its activity was increased by 47 and 50 %, in the presence of 10 % (v/v) ethanol and isopropanol, respectively. The new lipase AFL67 highly enantioselectively deacylated (S)-α-acetoxyphenylacetic acid (APA) and o-Cl-APA, m-Cl-APA, and p-Cl-APA to (S)-mandelic acid and its derivates. These features render this cold-active novel lipase AFL67 attractive for biotechnological applications in the field of enantioselective synthesis of chiral mandelic acids, o-acylated mandelic acids, and their derivates and detergent additives.







Similar content being viewed by others
References
Linhart, I. (2001). Stereochemistry of styrene biotransformation. Drug Metabolism Reviews, 33, 353–367.
Reetz, M. T. (2002). Lipases as practical biocatalysts. Current Opinion in Chemical Biology, 6, 145–150.
Santaniello, E., Ferraboschi, P., Grisenti, P., & Manzocchi, A. (1992). The biocatalytic approach to the preparation of enantiomerically pure chiral building blocks. Chemical Reviews, 92, 1071–1140.
Utkin, I. B., Yakimov, M. M., Matveeva, L. N., et al. (1991). Degradation of styrene and ethylbenzene by Pseudomonas species Y2. FEMS Microbiology Letters, 77, 237–241.
Ghanem, A., Aboul-Enein, M. N., El-Azzouny, A., & El-Behairy, M. F. (2010). Lipase-mediated enantioselective kinetic resolution of racemic acidic drugs in non-standard organic solvents: direct chiral liquid chromatography monitoring and accurate determination of the enantiomeric excesses. Journal of Chromatography. A, 1217, 1063–1074.
Blay, G., Fernandez, I., Molina, E., et al. (2006). Diastereoselective Michael addition of (S)-mandelic acid enolate to 2-arylidene-1,3-diketones: enantioselective diversity-oriented synthesis of densely substituted pyrazoles. Tetrahedron, 62, 8069–8076.
Mateo, C., Chmura, A., Rustler, S., et al. (2006). Synthesis of enantiomerically pure (S)-mandelic acid using an oxynitrilase-nitrilase bienzymatic cascade: a nitrilase surprisingly shows nitrile hydratase activity. Tetrahedron-Asymmetry, 17, 320–323.
Ema, T., Ide, S., Okita, N., & Sakai, T. (2008). Highly efficient chemoenzymatic synthesis of methyl (R)-o-chloromandelate, a key intermediate for clopidogrel, via asymmetric reduction with recombinant Escherichia coli. Advanced Synthesis and Catalysis, 350, 2039–2044.
Grimley, J. (2006). Pharma challenged. Chemical & Engineering News, 84, 17–28.
Wang, P. Y., Tsai, S. W., & Chen, T. L. (2008). Improvements of enzyme activity and enantioselectivity via combined substrate engineering and covalent immobilization. Biotechnology and Bioengineering, 101, 460–469.
Kim, E. K., Jang, W. H., Ko, J. H., et al. (2001). Lipase and its modulator from Pseudomonas sp strain KFCC 10818: proline-to-glutamine substitution at position 112 induces formation of enzymatically active lipase in the absence of the modulator. Journal of Bacteriology, 183, 5937–5941.
Gill, I. I., Das, J., & Patel, R. N. (2007). Enantioselective enzymatic acylation of 1-(3 ′-bromophenyl)ethylamine. Tetrahedron-Asymmetry, 18, 1330–1337.
Ismail, H., Lau, R. M., van Rantwijk, F., & Sheldon, R. A. (2008). Fully enzymatic resolution of chiral amines: acylation and deacylation in the presence of Candida antarctica lipase B. Advanced Synthesis and Catalysis, 350, 1511–1516.
Pilissao, C., Carvalho, P. D., & Nascimento, M. D. (2009). Enantioselective acylation of (RS)-phenylethylamine catalysed by lipases. Process Biochemistry, 44, 1352–1357.
Wang, Y. H., Li, Q. S., Zhang, Z. M., Ma, J. T., & Feng, Y. (2009). Solvent effects on the enantioselectivity of the thermophilic lipase QLM in the resolution of (R, S)-2-octanol and (R, S)-2-pentanol. Journal of Molecular Catalysis B: Enzymatic, 56, 146–150.
Ghanem, A., & Aboul-Enein, H. Y. (2005). Application of lipases in kinetic resolution of racemates. Chirality, 17, 1–15.
Cabrera, Z., Fernandez-Lorente, G., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2009). Enhancement of Novozym-435 catalytic properties by physical or chemical modification. Process Biochemistry, 44, 226–231.
Volpato, G., Filice, M., Rodrigues, R. C., et al. (2009). Modulation of a lipase from Staphylococcus warneri EX17 using immobilization techniques. Journal of Molecular Catalysis B: Enzymatic, 60, 125–132.
Ju, X., Yu, H.-L., Pan, J., Wei, D.-Z., & Xu, J.-H. (2009). Bioproduction of chiral mandelate by enantioselective deacylation of α-acetoxyphenylacetic acid using whole cells of newly isolated Pseudomonas sp. ECU1011. Applied Microbiology and Biotechnology, 86, 83–91.
Mhetras, N., Bastawde, K., & Gokhale, D. (2009). Purification and characterization of acidic lipase from Aspergillus niger NCIM 1207. Bioresource Technology, 100, 1486–1490.
Rajan, A., Kumar, D. R. S., & Nair, A. J. (2011). Isolation of a novel alkaline lipase producing fungus Aspergillus fumigatus MTCC 9657 from aged and crude rice bran oil and quantification by HPTLC. International Journal of Biological Chemistry, 5, 116–126.
Shi, B. L., Zeng, L. Q., Song, H. L., et al. (2010). Cloning and expression of Aspergillus tamarii FS132 lipase gene in Pichia pastoris. International Journal of Molecular Sciences, 11, 2373–2382.
Nishikawa, M., Gomi, H., & Kishimoto, F. (1993). Purification and some properties of carboxyl esterase from a Arthrobacter globiformis: stereoselective hydrolysis of ethyl chrysanthemate. Bioscience, Biotechnology, and Biochemistry, 57, 594–598.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72, 248–254.
Wang, Y., Zhao, J., Xu, J.-H., et al. (2010). Significantly improved expression and biochemical properties of recombinant Serratia marcescens lipase as robust biocatalyst for kinetic resolution of chiral ester. Applied Biochemistry and Biotechnology, 162, 2387–2399.
Becker, P., Abu-Reesh, I., Markossian, S., Antranikian, G., & Markl, H. (1997). Determination of the kinetic parameters during continuous cultivation of the lipase-producing thermophile Bacillus sp. IHI-91 on olive oil. Applied Microbiology and Biotechnology, 48, 184–190.
Contesini, F. J., Lopes, D. B., Macedo, G. A., Nascimento, M. D. G., & Carvalho, P. D. O. (2010). Aspergillus sp. lipase: potential biocatalyst for industrial use. Journal of Molecular Catalysis B: Enzymatic, 67, 163–171.
de Oliveira Carvalho, P., Contesini, F., Bizaco, R., & Alves Macedo, G. (2005). Kinetic properties and enantioselectivity of the lipases produced by four Aspergillus species. Food Biotechnology, 19, 183–192.
Gupta, R., Gupta, N., & Rathi, P. (2004). Bacterial lipases: an overview of production, purification and biochemical properties. Applied Microbiology and Biotechnology, 64, 763–781.
Zheng, X., Chu, X., Zhang, W., Wu, N., & Fan, Y. (2011). A novel cold-adapted lipase from Acinetobacter sp. XMZ-26: gene cloning and characterisation. Applied Microbiology and Biotechnology, 90, 971–980.
Hiol, A., Jonzo, M. D., Rugani, N., et al. (2000). Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzyme and Microbial Technology, 26, 421–430.
Zhang, A., Gao, R., Diao, N., et al. (2009). Cloning, expression and characterization of an organic solvent tolerant lipase from Pseudomonas fluorescens JCM5963. Journal of Molecular Catalysis B: Enzymatic, 56, 78–84.
Cabrera, Z., Fernandez-Lorente, G., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2009). Novozym 435 displays very different selectivity compared to lipase from Candida antarctica B adsorbed on other hydrophobic supports. Journal of Molecular Catalysis B: Enzymatic, 57, 171–176.
Palomo, J. M., Fernandez-Lorente, G., Mateo, C., et al. (2002). Modulation of the enantioselectivity of lipases via controlled immobilization and medium engineering: hydrolytic resolution of mandelic acid esters. Enzyme and Microbial Technology, 31, 775–783.
Acknowledgments
This research was financially supported by China National Special Fund for State Key Laboratory of Bioreactor Engineering (no. 2060204) and Shanghai Leading Academic Discipline Project (no. B505).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiao-Jiao Shangguan and Li-qiang Fan contributed equally to this work
Rights and permissions
About this article
Cite this article
Shangguan, JJ., Fan, Lq., Ju, X. et al. Expression and Characterization of a Novel Enantioselective Lipase from Aspergillus fumigatus . Appl Biochem Biotechnol 168, 1820–1833 (2012). https://doi.org/10.1007/s12010-012-9899-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9899-x