Abstract
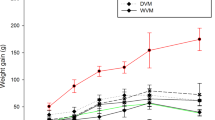
The effects of the dietary addition of 2.5 % (w/w) Amaranthus mantegazzianus protein isolate (AI) on blood pressure, lipid profiles and antioxidative status of Wistar rats were evaluated. Six diets were used to feed animals during 28 days: (base (AIN93G), Chol (cholesterol 1 %, w/w), CE (α-tocopherol 0.005 %, w/w), CholE (cholesterol 1 % (w/w) + α-tocopherol 0.005 %, w/w), CAI (AI 2.5 % w/w), CholAI (cholesterol 1 % (w/w) + AI 2.5 %, w/w). Lipid profiles of plasma and liver and faecal cholesterol content were analyzed. Antioxidant status was evaluated by the ferric reducing activity of plasma (FRAP), the 2-thiobarbituric acid (TBA) assay and superoxide dismutase (SOD) activity in plasma and liver. Blood pressure was measured in the tail artery of rats. CholA group presented a significant (α < 0.05) reduction (16 %) in the plasma total cholesterol. In liver, the intake of cholesterol (Chol group) induced a significant increment in cholesterol and triglycerides (2.5 and 2.3 times, respectively), which could be decreased (18 % and 47 %, respectively) by the addition of AI (CholA group). This last group also showed an increased faecal cholesterol excretion (20 %). Increment (50 %) in FRAP values, diminution of TBA value in plasma and liver (70 % and 38 %, respectively) and diminution of SOD activity (20 %) in plasma of CholA group suggest an antioxidant effect because of the intake of AI. In addition, CA and CholA groups presented a diminution (18 %) of blood pressure after 28 days.


Similar content being viewed by others
References
Vecchi B, Añón M (2009) ACE inhibitory tetrapeptides from Amaranthus hypochondriacus 11S globulin. Phytochem. 70(7):864–870
Fritz M, Vecchi B, Rinaldi G, Añón M (2011) Amaranth seed protein hydrolysates have in vivo and in vitro antihypertensive activity. Food Chem 126:878–884
Escudero N, Zirulnik F, Gomez N, Mucciarelli S, Giménez M (2006) Influence of a protein concentrate from Amaranthus cruentus seeds on lipid metabolism. Exp Biol Med 231:50–59
Mendonça S, Saldiva P, Cruz R, Arêas A (2009) Amaranth protein presents cholesterol-lowering effect. Food Chem 116:738–742
Nsimba R, Kikuzaki H, Konishi Y (2008) Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem 106:760–766
López V, Razzeto G, Giménez M, Escudero N (2011) Antioxidant properties of A. hypochondriacus seeds and their effect on the liver of alcohol-treated rats. Plant Foods Hum Nutr 66:157–162
Orsini Delgado M, Tironi V, Añón M (2011) Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT-Food Sci Technol 44:1752–1760
Orsini Delgado M, Galleano M, Añón M, Tironi V (2015) Amaranth peptides from gastrointestinal digestion: antioxidant activity against physiological reactive species. Plant Foods Human Nutr 70:27–34
Martinez N, Añón MC (1996) Composition and structural characterization of amaranth protein isolates. An electrophoretic and calorimetric study. J Agric Food Chem 44:2523–2530
Reeves P, Nielsen F, Fahey Jr G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Diet J Nutr 123:1939–1951
Friedewald W, Levy R, Fredrickson D (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Chen G, Luo Y, Ji B, Li B, Su W, Xiao Z, Zhang G (2011) Hypocolesterolemic effects of Auricularia auricula ethanol extract in ICR mice fed a cholesterol-enriched diet. J Food Sci Technol 48(6):692–698
Bragagnolo N, Rodríguez-Amaya D (2003) Comparison of the cholesterol content of Brazilian chicken and quail eggs. J Food Composition Anal 16:147–153
Benzie I, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Yagi K (1976) A simple fluorometric assay for lipoperoxides in blood plasma. Biochem Med 15:121–216
Friesen J, Rodwell, V. (2004). The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biology, 5, 11, Article 248.
Fritz M, Rinaldi G (2007) Influence of nitric oxide-mediated vasodilation on the blood pressure measured with the tail-cuff method in the rat. J Biomed Sci 14(6):757–765
Czerwinski J, Bartnikowska E, Leontowicz H, Langeb E, Leontowicz M, Katrich E, Trakhtenbergd S, Gorinstein S (2004) Oat (Avena sativa L.) and amaranth (Amaranthus hypochondriacus) meals positively affect plasma lipid profile in rats fed cholesterol containing diets. J Nutr Biochem 15:622–629
Oschry Y, Eisenberg S (1982) Rat plasma lipoproteins: re-evaluation of a lipoprotein system devoid of cholestryl ester transfer activity. J Lipid Res 23:1099–1106
Christie W (1985) Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J Lipid Res 26:507–512
Itou K, Akahane Y (2010) Effect of extracts from narezushi, a fermented mackerel product, on cholesterol metabolism in wistar rats. J Food Sci Technol 76:537–546
Frota K, Mendonça S, Saldiva R, Cruz R, Arêas J (2008) Cholesterol-lowering properties of whole cowpea seed and its protein isolate in hamsters. J. Food Sci 73(9):235–240
Roach P, Balasubramaniam S, Hirata F, Abbey M, Szanto A, Simons L, Nestel P (1993) The low-density lipoprotein receptor and cholesterol synthesis are affected differently by dietary cholesterol in the rat. Biochim Biophys Acta 1170:165–172
Qureshi A, Lehmann J, Peterson D (1996) Amaranth and its oil inhibit cholesterol biosynthesis in 6-week-old female chickens. J Nutr 126:1972–1978
Howard A, Udenigwe C (2013) Mechanisms and prospects of food protein hydrolysates and peptide-induced hypolidaemia. Food Funct 4:40–51
Kok F, van Poppel G, Melse J, Verheul E, Schouten E, Kruyssen D, Hofman A (1991) Do antioxidants and polyunsaturated fatty acids have a combined association with coronary atherosclerosis? Atherosclerosis 86(1):85–90
Prasad K, Kalra J (1989) Experimental atherosclerosis and oxygen free radicals. Angiology 40:835–843
Gupta V, Lahiri S, Sultana S, Tulsawani T, Kumar R (2010) Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C–H–R) exposure and post-stress recovery. Food Chem Toxicol 48:1019–1025
Tsai C (1975) Lipid peroxidation and glutathione peroxidase activity in the liver of cholesterol-fed rats. J Nutr 105:946–951
Wang D, Wang L, Zhu F, Zhu J, Chen X, Zou L, Saito M, Li L (2008) In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of douchi (a traditional Chinese salt-fermented soybean food). Food Chem 107:1421–1428
Nazeer R, Kumar N, Ganesh R (2012) In vitro and in vivo studies on the antioxidant activity of fish peptide isolated from the croaker (Otolithes ruber) muscle protein hydrolysate. Peptides 35:261–268
Acknowledgments
This work has been supported by an Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina) project (PICT 2007-1119) and a CONICET project (PIP 2008-01330). Authors Rinaldi, Añón and Tironi are members of CONICET (Argentina). Authors want to thank to Nilda Reinaudi (Facultad de Agronomía, UNLPam, Argentina) and Guillermo Peiretti (Facultad de Agronomía, UNRC, Argentina) for the provision of amaranth seeds, and to Cátedra de Nutrición y Bromatología (Facultad de Farmacia y Bioquímica, UBA, Argentina) for the collaboration in the diets preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
M. B. Lado declares that she has no conflict of interest.
J. Burini declares that she has no conflict of interest.
G. Rinaldi declares that she has no conflict of interest.
M. C. Añón declares that she has no conflict of interest.
V. Tironi declares that she has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Lado, M.B., Burini, J., Rinaldi, G. et al. Effects of the Dietary Addition of Amaranth (Amaranthus mantegazzianus) Protein Isolate on Antioxidant Status, Lipid Profiles and Blood Pressure of Rats. Plant Foods Hum Nutr 70, 371–379 (2015). https://doi.org/10.1007/s11130-015-0516-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0516-3