Abstract
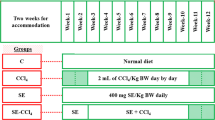
Amaranth constitutes a valuable pseudocereal, due to its nutritional quality and its nutraceutical properties, which contribute to improve human health. This work evaluated the effect of a diet based on Amaranthus hypochondriacus (Ah) seed on oxidative stress and antioxidant status in the liver of rats sub-chronically exposed to ethanol. The seed extract was investigated for antioxidant capacity in vitro, showing an adequate content of total phenols and antioxidant activity elevated. For in vivo assays, four groups of six rats each were fed with an AIN-93 M diet for 28 days. In groups III and IV casein was replaced by Ah as the protein source; groups II and IV were received ethanol in the drinking water (20% v/v). When comparing groups IV and II, the following was observed: significant decrease in the activity of aspartate aminotransferase and content of malondialdehyde (p < 0.001) in serum; decrease of malondialdehyde and increase in the activity and gene expression of Cu,Zn-superoxide dismutase, also, decrease in the NADPH oxidase transcript levels (p < 0.05) in liver. Our data suggest that Ah is a good source of total phenols and exerts a protective effect in serum and in liver of rats intoxicated with ethanol.

Similar content being viewed by others
Abbreviations
- ADH:
-
alcohol dehydrogense
- Ah :
-
Amaranthus hypochondriacus
- ALP:
-
alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BHT:
-
Buthylated hydroxy toluene
- CAT:
-
Catalase
- CYP2E1:
-
Cytochrome P450-2E1
- DNPH:
-
2,4-dinitrophenylhidrazine
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- GGT:
-
Gamma glutamyl transferase
- GPx:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
- M-MLV:
-
Moloney Murine Leukemia Virus Reverse Transcriptase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NO:
-
Nitric oxide
- NO test:
-
Scavenging activity against nitric oxide
- NOX:
-
NADPH oxidase
- PCR:
-
Polymerase chain reaction
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- RSA:
-
Radical scavenging activity
- RT:
-
Reverse transcription
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric Acid Reactive Substances
- TMP:
-
1,1,3,3-tetramethoxypropane
References
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Cuevas-Rodríguez EO, Dia VP, Yousef GG, García-Saucedo PA, López-Medina J, Paredes-López O, Gonzalez de Mejia E, Lila MA (2010) Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J Agric Food Chem 58(17):9542–9548
Nanji AA, Griniuviene B, Sadrzadeh SM, Levitsky S, McCully JD (1995) Effect of type of dietary fat and ethanol on antioxidant enzyme mRNA induction in rat liver. J Lipid Res 36(4):736–744
Liu RH (2003) Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr 78:517S–520S
Bressani R (2003) Amaranth. In: Caballero B (ed), Encyclopedia of Food Sciences and Nutrition, 2nd edn. Elsevier, Maryland, pp 166–173
Pedersen HA (2010) Synthesis and quantitation of six phenolic amides in Amaranthus spp. J Agric Food Chem 58:6306–6311
Alvarez-Jubete L, Arendt EK, Gallagher E (2009) Nutritive value of pseudocereals and their increasing use as functional gluten free ingredients. Int J Food Sci Nutr 60(4):240–257
Vinson JA, Proch J, Bose P (2001) Determination of the quantity and quality of polyphenol antioxidants in foods and beverages. Methods Enzymol 335:103–114
Emmons CL, Peterson DM, Paul GL (1999) Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. J Agric Food Chem 47:4894–4898
Eberhardt MV, Lee CY, Liu RH (2000) Antioxidant activity of fresh apples. Nature 405:903–904
Cheng GW, Breen PJ (1991) Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci 116(5):865–869
Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Gardès-Albert M (1994) Antioxidant action of Ginkgo biloba extracts EGb 761. Methods Enzymol 234:462–475
Cuendet M, Hostettmann K, Potterat O, Dyatmiko W (1997) Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta 80(4):1144–1152
Burits M, Bucar F (2000) Antioxidant activity of Nigella sativa essential oil. Phytother Res 14:323–328
Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal 13:8–17
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 Purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Vengeliene V, Vollmayr B, Henn FA, Spanagel R (2005) Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology 178(2–3):125–132
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Reznick AZ, Packer L (1994) Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein. J Biol Chem 244:6049–6055
Flohé L, Otting F (1984) Superoxide assays. Methods Enzymol 105:93–104
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Snedecor GW, Cochran WG (1980) Statistical Methods, 7th edn. Iowa State University Press, Ames
Paredes-López O, Cervantes-Ceja ML, Vigna-Pérez M, Hernández-Pérez T (2010) Berries: Improving human health and healthy aging, and promoting quality life: a review. Plant Foods Hum Nutr 65:299–308
Czerwinski J, Bartnikowska E, Leontowicz H, Lange E, Leontowicz M, Katrich E, Trakhtenberg S, Gorinstein S (2004) Oat (Avena sativa L.) and amaranth (Amaranthus hypochondriacus) meals positively affect plasma lipid profile in rats fed cholesterol containing diets. J Nutr Biochem 15:622–629
Taylor LP, Briggs WR (1990) Genetic regulation and photocontrol of anthocyanin accumulation in maize seedlings. Plant Cell 2:115–127
Dube A, Bharti S, Laloraya MM (1992) Inhibition of anthocyanin synthesis by cobaltous ions in the first internode of Sorghum bicolor L. Moench. J Exp Bot 43(10):1379–1382
Nsimba RY, Kikuzaki H, Konishi Y (2008) Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem 106(2):760–766
McDonough KH (2003) Antioxidant nutrients and alcohol. Toxicology 189:89–97
Dalle Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406
Morifuji M, Aoyama Y (2002) Dietary orotic acid affects antioxidant enzyme mRNA levels and oxidative damage to lipids and proteins in rat liver. J Nutr Biochem 13:403–410
Eom S-Y, Zhang YW, Ogawa M, Oyama T, Isse T, Kang J-W, Lee C-J, Kim Y-D, Kawamoto T, Kim H (2007) Activities of antioxidant enzymes induced by ethanol exposure in aldehyde dehydrogenase 2 knockout mice. J Health Sci 53(4):378–381
Acknowledgments
We are grateful to Engineer Guillermo Peiretti (Professor of the Agronomical and Veterinary Sciences Department National University of Rio Cuarto) for kindly providing the seeds employed in this work, obtained as an original variety from an experimental cultivation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucero López, V.R., Razzeto, G.S., Giménez, M.S. et al. Antioxidant Properties of Amaranthus hypochondriacus Seeds and their Effect on the Liver of Alcohol-Treated Rats. Plant Foods Hum Nutr 66, 157–162 (2011). https://doi.org/10.1007/s11130-011-0218-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-011-0218-4