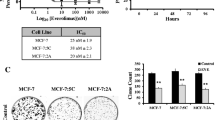
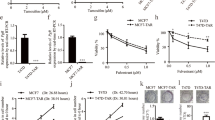
Abstract
Progression or recurrence due to resistance to aromatase inhibitors (AIs) is a significant clinical problem for a considerable number of patients with breast cancer. Programmed cell death 4 (PDCD4), a tumor suppressor protein, is targeted for degradation during tumor progression. In the current study, we aimed to examine PDCD4 expression and regulation in AI-resistant breast cancer cells, and its association with survival in patients with estrogen receptor (ER)-positive breast cancer. We determined PDCD4 expression levels in AI-resistant breast cancer cell lines and ER-positive breast cancer tumors, investigated the regulation of PDCD4 in AI-resistant breast cancer cell lines, and carried out a Kaplan–Meier survival analysis in two independent cohorts that included a total of 420 patients with ER-positive breast cancer. We found that PDCD4 expression was down-regulated in AI-resistant breast cancer cells, and this down-regulation was inversely correlated with activation of HER2 signaling. Moreover, lower expression of PDCD4 was significantly associated with HER2 positive status in ER-positive breast tumors. Down-regulation of PDCD4 was mediated through up-regulation of HER2 via the mitogen-activated protein kinase (MAPK), protein kinase B (PKB/AKT), and miR-21 in AI-resistant breast cancer cells. MiR-21 inhibitor and the ER down-regulator fulvestrant induced PDCD4 expression and decreased cell proliferation in AI-resistant breast cancer cells. Furthermore, forced overexpression of PDCD4 resensitized AI-resistant cells to AI or hormone deprivation. Finally, we identified that down-regulation of PDCD4 was associated with a lower rate of disease-free survival in patients with ER-positive breast cancer and high histologic grade of breast tumors. In summary, our study shows that expression of PDCD4 is down-regulated by HER2 signaling in AI-resistant breast cancer. Down-regulation of PDCD4 is associated with AI resistance and a poor prognosis in patients with ER-positive breast cancer.






Similar content being viewed by others
Abbreviations
- AI:
-
Aromatase inhibitor
- AKT/PKB:
-
Protein kinase B
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- MAPK:
-
Mitogen-activated protein kinase
- MTT:
-
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PDCD4:
-
Programmed cell death 4
- PI3K:
-
Phosphatidylinositol 3′-kinase
- PTEN:
-
Phosphatase and tensin homolog
- SiRNA:
-
Short interfering RNA
- TCGA:
-
The cancer genome atlas
References
Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP (2006) Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 98(18):1285–1291. doi:10.1093/jnci/djj357
Nemere I, Pietras RJ, Blackmore PF (2003) Membrane receptors for steroid hormones: signal transduction and physiological significance. J Cell Biochem 88(3):438–445. doi:10.1002/jcb.10409
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H (2001) Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 276(13):9817–9824. doi:10.1074/jbc.M010840200
Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P (1995) Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270(5241):1491–1494
Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278(33):30458–30468. doi:10.1074/jbc.M305226200
Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM (2005) Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res 65(12):5380–5389. doi:10.1158/0008-5472.CAN-04-4502
Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, Morita M, Kakeji Y, Baba H, Maehara Y (2006) Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer 13(2):137–144
Flageng MH, Moi LL, Dixon JM, Geisler J, Lien EA, Miller WR, Lonning PE, Mellgren G (2009) Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br J Cancer 101(8):1253–1260. doi:10.1038/sj.bjc.6605324
Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T (1995) Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 166(2):297–301
Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, Matsuhashi S, Colburn NH (1999) Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci U S A 96(24):14037–14042
Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH (2003) The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 23(1):26–37
Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH (2004) A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol 24(9):3894–3906
Jansen AP, Camalier CE, Colburn NH (2005) Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 65(14):6034–6041. doi:10.1158/0008-5472.CAN-04-2119
Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH (2008) Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res 68(5):1254–1260. doi:10.1158/0008-5472.CAN-07-1719
Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, Ozaki I, Petersen I (2003) Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol 200(5):640–646. doi:10.1002/path.1378
Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, Post S, Jansen A, Colburn NH, Allgayer H (2007) Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 110(8):1697–1707. doi:10.1002/cncr.22983
Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S (2006) Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25(45):6101–6112. doi:10.1038/sj.onc.1209634
Wen YH, Shi X, Chiriboga L, Matsahashi S, Yee H, Afonja O (2007) Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol Rep 18(6):1387–1393
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133(2):647–658. doi:10.1053/j.gastro.2007.05.022
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283(2):1026–1033. doi:10.1074/jbc.M707224200
Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y (2008) MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27(31):4373–4379. doi:10.1038/onc.2008.72
Meric-Bernstam F, Chen H, Akcakanat A, Do KA, Lluch A, Hennessy BT, Hortobagyi GN, Mills GB, Gonzalez-Angulo A (2012) Aberrations in translational regulation are associated with poor prognosis in hormone receptor-positive breast cancer. Breast Cancer Res 14(5):R138. doi:10.1186/bcr3343
Zhou DJ, Pompon D, Chen SA (1990) Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res 50(21):6949–6954
Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S (2008) Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res 68(12):4910–4918. doi:10.1158/0008-5472.CAN-08-0303
Wong C, Wang X, Smith D, Reddy K, Chen S (2012) AKT-aro and HER2-aro, models for de novo resistance to aromatase inhibitors; molecular characterization and inhibitor response studies. Breast Cancer Res Treat 134(2):671–681. doi:10.1007/s10549-012-2105-6
Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X (2015) Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Mol Cell Endocrinol 399:32–42. doi:10.1016/j.mce.2014.09.016
Spandidos A, Wang X, Wang H, Seed B (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucl Acids Res 38:792–799. doi:10.1093/nar/gkp1005
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Team RC (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, Wong JE, Liu ET, Bergh J, Kuznetsov VA, Miller LD (2006) Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res 66(21):10292–10301. doi:10.1158/0008-5472.CAN-05-4414
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365(9460):671–679. doi:10.1016/S0140-6736(05)17947-1
Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M (2006) S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314(5798):467–471. doi:10.1126/science.1130276
Huang TH, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A, McManus MT (2009) Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem 284(27):18515–18524. doi:10.1074/jbc.M109.006676
Lee JA, Lee HY, Lee ES, Kim I, Bae JW (2011) Prognostic implications of MicroRNA-21 overexpression in invasive ductal carcinomas of the breast. J Breast Cancer 14(4):269–275. doi:10.4048/jbc.2011.14.4.269
Martin LA, Farmer I, Johnston SR, Ali S, Dowsett M (2005) Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocr Relat Cancer 12(Suppl 1):S75–S84. doi:10.1677/erc.1.01023
Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J Jr, Berstein L, Yue W (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Suppl 1):S61–S73. doi:10.1677/erc.1.01018
Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH (2004) Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 23(49):8135–8145. doi:10.1038/sj.onc.1207983
Lankat-Buttgereit B, Goke R (2009) The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell 101(6):309–317. doi:10.1042/BC20080191
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2(2):127–137. doi:10.1038/35052073
Stephen RL, Shaw LE, Larsen C, Corcoran D, Darbre PD (2001) Insulin-like growth factor receptor levels are regulated by cell density and by long term estrogen deprivation in MCF7 human breast cancer cells. J Biol Chem 276(43):40080–40086. doi:10.1074/jbc.M105892200
Fox EM, Miller TW, Balko JM, Kuba MG, Sanchez V, Smith RA, Liu S, Gonzalez-Angulo AM, Mills GB, Ye F, Shyr Y, Manning HC, Buck E, Arteaga CL (2011) A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res 71(21):6773–6784. doi:10.1158/0008-5472.CAN-11-1295
Gilani RA, Kazi AA, Shah P, Schech AJ, Chumsri S, Sabnis G, Jaiswal AK, Brodie AH (2012) The importance of HER2 signaling in the tumor-initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat 135(3):681–692. doi:10.1007/s10549-012-2148-8
Roop RP, Ma CX (2012) Endocrine resistance in breast cancer: molecular pathways and rational development of targeted therapies. Future Oncol 8(3):273–292. doi:10.2217/fon.12.8
Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM (2009) Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells (Nucleic acids research). Nucl Acids Res 37(8):2584–2595. doi:10.1093/nar/gkp117
Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ (2001) Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res 61(16):5985–5991
Maruani DM, Spiegel TN, Harris EN, Shachter AS, Unger HA, Herrero-Gonzalez S, Holz MK (2012) Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene 31(49):5073–5080. doi:10.1038/onc.2011.657
Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM (2013) Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45(12):1446–1451. doi:10.1038/ng.2823
Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R, Yelensky R, Brown M, Miller VA, Sarid D, Rizel S, Klein B, Rubinek T, Wolf I (2013) D538G mutation in estrogen receptor-alpha: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 73(23):6856–6864. doi:10.1158/0008-5472.CAN-13-1197
Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M (2010) MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 285(26):20281–20290. doi:10.1074/jbc.M110.109207
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH (2011) MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS ONE 6(4):e19139. doi:10.1371/journal.pone.0019139
Masri S, Liu Z, Phung S, Wang E, Yuan YC, Chen S (2010) The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat 124(1):89–99. doi:10.1007/s10549-009-0716-3
Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, Vagner S (2009) Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res 69(21):8332–8340. doi:10.1158/0008-5472.CAN-09-2206
Hayes EL, Lewis-Wambi JS (2015) Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res 17:40. doi:10.1186/s13058-015-0542-y
Acknowledgments
The study was supported by the National Institutes of Health (R01 ES08258 to SC). Research reported in this publication included work performed at the Bioinformatics Core supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Yuan, YC., Wang, Y. et al. Down-regulation of programmed cell death 4 (PDCD4) is associated with aromatase inhibitor resistance and a poor prognosis in estrogen receptor-positive breast cancer. Breast Cancer Res Treat 152, 29–39 (2015). https://doi.org/10.1007/s10549-015-3446-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3446-8