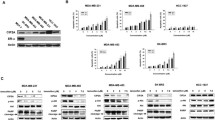
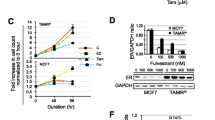
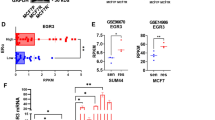
Abstract
Purpose
Endocrine therapy is the most commonly used approach for the treatment of estrogen receptor (ERα)-positive breast cancer. The cure rate of patients with ERα-positive breast cancer is, however, limited due to the occurrence of endocrine resistance. Loss of ERα is one major mechanism for the occurrence of endocrine resistance. Recent studies have shown that pan-HDAC inhibitors may be effective in reversing endocrine resistance. However, the molecular mechanism underlying this reversal has remained largely unknown. Here we aimed to unravel this mechanism.
Methods
Endocrine resistant breast cancer cell lines were established through exposure to tamoxifen. mRNA expression was assessed by qRT-PCR and protein expression by Western blotting. The effect of HDAC3 inhibition on the viability of endocrine resistant breast cancer cells was evaluated using CCK-8 and colony forming assays. Immunohistochemistry was used to detect protein expression in primary breast cancer tissues.
Results
We found that in endocrine resistant breast cancer cells loss of ERα led to HDAC3 stabilization via decreased ERα-mediated caspase7 expression, resulting in reduced caspase7-mediated HDAC3 cleavage. We also found that the ERα-caspase7-HDAC3 axis determined the global H3K9 and H4K16 acetylation status, which was positively correlated with ERα expression. Finally, we found that inhibition of HDAC3 significantly decreased the viability of endocrine resistant breast cancer cells exhibiting ERα deficiency. The ERα-caspase7-HDAC3 axis was subsequently verified in primary endocrine resistant breast cancer samples.
Conclusions
From our data we conclude that the ERα-caspase7-HDAC3 axis may play a role in promoting the proliferation of endocrine resistant breast cancer cells. HDAC3 may serve as a therapeutic target for (a subset of) endocrine resistant breast cancers exhibiting ERα loss.







Similar content being viewed by others
References
T. De Marchi, J.A. Foekens, A. Umar, J.W. Martens, Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov Today 21, 1181–1188 (2016)
A.B. Castrellon, Novel strategies to improve the endocrine therapy of breast Cancer. Oncol Rev 11, 323 (2017)
C. Busonero, S. Leone, F. Acconcia, Emetine induces estrogen receptor alpha degradation and prevents 17beta-estradiol-induced breast cancer cell proliferation. Cell Oncol 40, 299–301 (2017)
S. Moverare-Skrtic, A.E. Borjesson, H.H. Farman, K. Sjogren, S.H. Windahl, M.K. Lagerquist, A. Andersson, A. Stubelius, H. Carlsten, J.A. Gustafsson, C. Ohlsson, The estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor alpha AF-2 is modified. Proc Natl Acad Sci USA. 111, 1180–1185 (2014)
S.J. Howell, S.R. Johnston, A. Howell, The use of selective estrogen receptor modulators and selective estrogen receptor down-regulators in breast cancer. Best Pract Res Clin Endocrinol Metab 18, 47–66 (2004)
R.I. Nicholson, J.M. Gee, D.L. Manning, A.E. Wakeling, M.M. Montano, B.S. Katzenellenbogen, Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann N Y Acad Sci 761, 148–163 (1995)
S. Bianco, N. Gevry, Endocrine resistance in breast cancer: From cellular signaling pathways to epigenetic mechanisms. Transcription. 3, 165–170 (2012)
F. Lumachi, G. Luisetto, S.M. Basso, U. Basso, A. Brunello, V. Camozzi, Endocrine therapy of breast cancer. Curr Med Chem 18, 513–522 (2011)
R. Schiff, S. Massarweh, J. Shou, C.K. Osborne, Breast cancer endocrine resistance: How growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 9, 447S–454S (2003)
G. Arpino, S.J. Green, D.C. Allred, D. Lew, S. Martino, C.K. Osborne, R.M. Elledge, HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin Cancer Res 10, 5670–5676 (2004)
J.M. Gee, J.F. Robertson, E. Gutteridge, I.O. Ellis, S.E. Pinder, M. Rubini, R.I. Nicholson, Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer 12(Suppl 1), S99–S111 (2005)
M. Dowsett, S. Johnston, L.A. Martin, J. Salter, M. Hills, S. Detre, M.C. Gutierrez, S.K. Mohsin, J. Shou, D.C. Allred, R. Schiff, C.K. Osborne, I. Smith, Growth factor signalling and response to endocrine therapy: The Royal Marsden Experience. Endocr Relat Cancer 12(Suppl 1), S113–S117 (2005)
A.J. Butt, C.M. McNeil, E.A. Musgrove, R.L. Sutherland, Downstream targets of growth factor and oestrogen signalling and endocrine resistance: The potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer 12(Suppl 1), S47–S59 (2005)
G. Pohl, M. Rudas, O. Dietze, S. Lax, E. Markis, R. Pirker, C.C. Zielinski, H. Hausmaninger, E. Kubista, H. Samonigg, R. Jakesz, M. Filipits, High p27Kip1 expression predicts superior relapse-free and overall survival for premenopausal women with early-stage breast cancer receiving adjuvant treatment with tamoxifen plus goserelin. J Clin Oncol 21, 3594–3600 (2003)
G. Perez-Tenorio, F. Berglund, A. Esguerra Merca, B. Nordenskjold, L.E. Rutqvist, L. Skoog, O. Stal, Cytoplasmic p21WAF1/CIP1 correlates with Akt activation and poor response to tamoxifen in breast cancer. J Clin Oncol 28, 1031–1042 (2006)
S.R. Johnston, G. Saccani-Jotti, I.E. Smith, J. Salter, J. Newby, M. Coppen, S.R. Ebbs, M. Dowsett, Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res 55, 3331–3338 (1995)
C.A. Encarnacion, D.R. Ciocca, W.L. McGuire, G.M. Clark, S.A. Fuqua, C.K. Osborne, Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat 26, 237–246 (1993)
M.C. Gutierrez, S. Detre, S. Johnston, S.K. Mohsin, J. Shou, D.C. Allred, R. Schiff, C.K. Osborne, M. Dowsett, Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 23, 2469–2476 (2005)
G. Deblois, V. Giguere, Oestrogen-related receptors in breast cancer: Control of cellular metabolism and beyond. Nat Rev Cancer 13, 27–36 (2013)
R. Autio, S. Kilpinen, M. Saarela, O. Kallioniemi, S. Hautaniemi, J. Astola, Comparison of Affymetrix data normalization methods using 6,926 experiments across five array generations. BMC Bioinformatics 10(Suppl 1), S24 (2009)
S. Kilpinen, R. Autio, K. Ojala, K. Iljin, E. Bucher, H. Sara, T. Pisto, M. Saarela, R.I. Skotheim, M. Bjorkman, J.P. Mpindi, S. Haapa-Paananen, P. Vainio, H. Edgren, M. Wolf, J. Astola, M. Nees, S. Hautaniemi, O. Kallioniemi, Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol 9, R139 (2008)
R.M. Neve, K. Chin, J. Fridlyand, J. Yeh, F.L. Baehner, T. Fevr, L. Clark, N. Bayani, J.P. Coppe, F. Tong, T. Speed, P.T. Spellman, S. DeVries, A. Lapuk, N.J. Wang, W.L. Kuo, J.L. Stilwell, D. Pinkel, D.G. Albertson, F.M. Waldman, F. McCormick, R.B. Dickson, M.D. Johnson, M. Lippman, S. Ethier, A. Gazdar, J.W. Gray, A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006)
L. Yin, X.T. Zhang, X.W. Bian, Y.M. Guo, Z.Y. Wang, Disruption of the ER-alpha36-EGFR/HER2 positive regulatory loops restores tamoxifen sensitivity in tamoxifen resistance breast cancer cells. PLoS One 9, e107369 (2014)
Y. Xia, J. Wang, T.J. Liu, W.K. Yung, T. Hunter, Z. Lu, c-Jun downregulation by HDAC3-dependent transcriptional repression promotes osmotic stress-induced cell apoptosis. Mol Cell 25, 219–232 (2007)
H.K. Choi, Y. Choi, E.S. Park, S.Y. Park, S.H. Lee, J. Seo, M.H. Jeong, J.W. Jeong, J.H. Jeong, P.C. Lee, K.C. Choi, H.G. Yoon, Programmed cell death 5 mediates HDAC3 decay to promote genotoxic stress response. Nat Commun 6, 7390 (2015)
S. Ropero, M. Esteller, The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 1, 19–25 (2007)
Y. Chervona, M. Costa, Histone modifications and cancer: Biomarkers of prognosis? Am J Cancer Res 2, 589–597 (2012)
S.E. Elsheikh, A.R. Green, E.A. Rakha, D.G. Powe, R.A. Ahmed, H.M. Collins, D. Soria, J.M. Garibaldi, C.E. Paish, A.A. Ammar, M.J. Grainge, G.R. Ball, M.K. Abdelghany, L. Martinez-Pomares, D.M. Heery, I.O. Ellis, Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res 69, 3802–3809 (2009)
J.H. Pickar, T. MacNeil, K. Ohleth, SERMs: Progress and future perspectives. Maturitas. 67, 129–138 (2010)
E.A. Musgrove, R.L. Sutherland, Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9, 631–643 (2009)
H. Kurokawa, A.E. Lenferink, J.F. Simpson, P.I. Pisacane, M.X. Sliwkowski, J.T. Forbes, C.L. Arteaga, Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60, 5887–5894 (2000)
Y. Liang, H. Han, L. Liu, Y. Duan, X. Yang, C. Ma, Y. Zhu, J. Han, X. Li, Y. Chen, CD36 plays a critical role in proliferation, migration and tamoxifen-inhibited growth of ER-positive breast cancer cells. Oncogenesis. 7, 98 (2018)
C.K. Osborne, R. Schiff, Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62, 233–247 (2011)
Y.K. Liang, Y.S. Zeng, Y.W. Xiao, Y.X. Ouyang, M. Chen, Y.C. Li, H.Y. Lin, X.L. Wei, Y.Q. Zhang, F.A. Kruyt, G.J. Zhang, MCAM/CD146 promotes tamoxifen resistance in breast cancer cells through induction of epithelialemesenchymal transition, decreased ERa expression and AKT activation. Cancer Lett 386, 65–76 (2017)
M.A. Glozak, E. Seto, Histone deacetylases and cancer. Oncogene. 26, 5420–5432 (2007)
C. Pilarsky, M. Wenzig, T. Specht, H.D. Saeger, R. Grutzmann, Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 6, 744–750 (2004)
Y. Ma, Y. Yue, M. Pan, J. Sun, J. Chu, X. Lin, W. Xu, L. Feng, Y. Chen, D. Chen, V.Y. Shin, X. Wang, H. Jin, Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am J Cancer Res 5, 663–673 (2015)
J. Fabian, M. Lodrini, I. Oehme, M.C. Schier, T.M. Thole, T. Hielscher, A. Kopp-Schneider, L. Opitz, D. Capper, A. von Deimling, I. Wiegand, T. Milde, U. Mahlknecht, F. Westermann, O. Popanda, F. Roels, B. Hero, F. Berthold, M. Fischer, A.E. Kulozik, O. Witt, H.E. Deubzer, GRHL1 acts as tumor suppressor in neuroblastoma and is negatively regulated by MYCN and HDAC3. Cancer Res 74, 2604–2616 (2014)
B.M. Muller, L. Jana, A. Kasajima, A. Lehmann, J. Prinzler, J. Budczies, K.J. Winzer, M. Dietel, W. Weichert, C. Denkert, Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer--overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 13, 215 (2013)
P.N. Munster, K.T. Thurn, S. Thomas, P. Raha, M. Lacevic, A. Miller, M. Melisko, R. Ismail-Khan, H. Rugo, M. Moasser, S.E. Minton, A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer 104, 1828–1835 (2011)
P. Raha, S. Thomas, K.T. Thurn, J. Park, P.N. Munster, Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res 17, 26 (2015)
M. Kirschbaum, P. Frankel, L. Popplewell, J. Zain, M. Delioukina, V. Pullarkat, D. Matsuoka, B. Pulone, A.J. Rotter, I. Espinoza-Delgado, A. Nademanee, S.J. Forman, D. Gandara, E. Newman, Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol 29, 1198–1203 (2011)
D.R. McIlwain, T. Berger, T.W. Mak, Caspase functions in cell death and disease. CSH Perspect Biol 5, a008656 (2013)
M. Brentnall, L. Rodriguez-Menocal, R.L. De Guevara, E. Cepero, L.H. Boise, Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14, 32 (2013)
T. Hashimoto, L. Yamauchi, T. Hunter, U. Kikkawa, S. Kamada, Possible involvement of caspase-7 in cell cycle progression at mitosis. Genes Cells 13, 609–621 (2008)
T. Hashimoto, U. Kikkawa, S. Kamada, Contribution of caspase(s) to the cell cycle regulation at mitotic phase. PLoS One 6, e18449 (2011)
S. Chaudhary, B. Madhukrishna, A.K. Adhya, S. Keshari, S.K. Mishra, Overexpression of caspase 7 is ERalpha dependent to affect proliferation and cell growth in breast cancer cells by targeting p21(Cip). Oncogenesis. 5, e219 (2016)
V.G. Allfrey, R. Faulkner, A.E. Mirsky, Acetylation and methylation of histones and their possible role in the regulation of Rna synthesis. Proc Natl Acad Sci USA. 51, 786–794 (1964)
B.G. Pogo, V.G. Allfrey, A.E. Mirsky, RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci USA. 55, 805–812 (1966)
L. Sealy, R. Chalkley, DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res 5, 1863–1876 (1978)
G. Vidali, L.C. Boffa, E.M. Bradbury, V.G. Allfrey, Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci USA 75, 2239–2243 (1978)
T.R. Hebbes, A.W. Thorne, C. Crane-Robinson, A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 7, 1395–1402 (1988)
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant No.: 81572712 & 81772956 to L. Chen), the National Basic Research Program of China (973 Program) (Grant No.: 2015CB965000 to L. Chen), the Key University Science Research Project of Jiangsu Province (Grant No.: 17KJA320002 to L. Chen), grants from the Natural Science Foundation of Jiangsu Province (Grant No.: SBK2016030027 to L. Chen), the Six talent peaks project in Jiangsu Province (Grant No.: 2015-JY-002 to L. Chen), a Jiangsu Shuangchuang talent program to L. Chen, and the Priority Academic Program Development of Jiangsu Higher Education Institutions. Science and Technology Program of Nanjing, China (Grant No.: 201803052 to Z. Yu).
Author information
Authors and Affiliations
Contributions
Conception and design: Shiyi Yu, Tao Zhu, Zhenghong Yu, Liming Chen.
Development of methodology: Liming Chen, Zhenghong Yu.
Acquisition of data (provided animals, acquired and managed patients, provided facilities): Shiyi Yu, Xue Gong, Zhifang Ma, Meng Zhang, Ling Huang, Jun zhang, Shuang Zhao, Zhenghong Yu, Liming Chen.
Analysis and interpretation of data (i.e., statistical analysis, biostatistics, computational analysis): Liming Chen, Shiyi Yu, Zhifang Ma.
Writing, review and/or revision of the manuscript: Liming Chen, Shiyi Yu.
Administrative, technical or material support (i.e., reporting or organizing data, constructing databases): Tao Zhu, Shuang Zhao, Zhenghong Yu.
Study supervision: Liming Chen.
Other (approved final version): Liming Chen.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
(PNG 41 kb)
High resolution image
(TIF 1895 kb)
Supplementary Table S1
(XLSX 8 kb)
Supplementary Table S2
(XLSX 8 kb)
Supplementary Table S3
(XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Yu, S., Gong, X., Ma, Z. et al. Endocrine resistant breast cancer cells with loss of ERα expression retain proliferative ability by reducing caspase7-mediated HDAC3 cleavage. Cell Oncol. 43, 65–80 (2020). https://doi.org/10.1007/s13402-019-00439-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00439-x