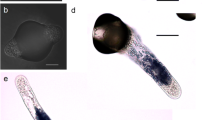
Abstract
The effect of different external factors on pollen germination and pollen tube growth is well documented for several species. On the other hand the consequences of these factors on the division of the generative nucleus and the formation of callose plugs are less known. In this study we report the effect of medium pH, 2-[N-morpholino]ethanesulfonic acid (MES) buffer, sucrose concentration, partial substitution of sucrose by polyethyleneglycol (PEG) 6000, arginine (Arg), and pollen density on the following parameters: pollen germination, pollen tube length, division of the generative nucleus, and the formation of callose plugs. We also studied the different developmental processes in relation to time. The optimal pH for all parameters tested was 6.7. In particular, the division of the generative nucleus and callose plug deposition were inhibited at lower pH values. MES buffer had a toxic effect; both pollen germination and pollen tube length were lowered. MES buffer also influenced migration of the male germ unit (MGU), the second mitotic division, and the formation of callose plugs. A sucrose concentration of 10% was optimal for pollen germination, pollen tube growth rate and final pollen tube length, as well as for division of the generative nucleus and the production of callose plugs. Partial substitution of sucrose by PEG 6000 had no influence on pollen germination and pollen tube length. However, in these pollen tubes the MGU often did not migrate and no callose plugs were observed. Pollen tube growth was independent of the migration of the MGU and the deposition of callose plugs. In previous experiments Arg proved to be positive for the division of the generative nucleus in pollen tubes cultured in vitro. Here, we found that more pollen tubes had callose plugs and more callose plugs per pollen tube were produced on medium with Arg. After the MGU migrated into the pollen tube (1 h after cultivation), callose plugs were deposited (3 h). After 8 h the first sperm cells were produced. The MGU moved away from the active pollen tube tip until the second pollen mitosis occurred, thereafter the distance from the MGU to the pollen tube tip diminished. Callose plug deposition never started prior to MGU migration into the pollen tube. Pollen tubes without a MGU also lack callose plugs (±30% of the total number of pollen tubes). Furthermore, we found a correlation between the occurrence of sperm cells in pollen tubes and the synthesis of callose plugs.




Similar content being viewed by others
References
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50:747–858
Brewbaker JL, Majumder SK (1961) Cultural studies of the pollen population effect and the self-incompatibility inhibition. Am J Bot 48:457–464
Cai G, Moscatelli A, Cresti M (1997) Cytoskeletal organization and pollen tube growth. Trends Plant Sci 2:86–91
Chen YF, Matsubayashi Y, Sakagami Y (2000) Peptide growth factor phytosulfokine-α contributes to the pollen population effect. Planta 211:752–755
Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15:133–139
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206:452–460
Fricker MD, NS White, G Obermeyer (1997) pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. J Cell Sci 110:1729–1740
Graaf BHJ de, Derksen JWM, Mariani C (2001) Pollen and pistil in the progamic phase. Sex Plant Reprod 14:41–55
Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1998) Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10:2019–2031
Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Paris C, Kunkel JG, Hepler PK (2003) Effect of extracellular calcium, pH and borate in Lilium formosanum pollen tubes. J Exp Bot 54:65–72
Holm SO (1994) Pollen density—effects on pollen germination and pollen tube growth in Betula pubescens Ehrh. in northern Sweden. New Phytol 126:541–547
Hudson LC, Stewart CN (2004) Effects of pollen-synthesized green fluorescent protein on pollen grain fitness. Sex Plant Reprod 17:49–53
Laitiainen E, Nieminen KM, Vihinen H, Raudakoski M (2002) Movement of the generative cell and vegetative nucleus in tobacco pollen tubes is dependent on microtubule cytoskeleton but independent of the synthesis of callose plugs. Sex Plant Reprod 15:195–204
Lalanne E, Twell D (2002) Genetic control of male germ unit organization in Arabidopsis. Plant Physiol 129:865–875
Li H, Bacic A, Read SM (1997) Activation of pollen tube callose synthase by detergents. Plant Physiol 114:1255–1265
Lubliner N, Singh-Cundy DT, Singh-Cundy A (2003) Characterization of the pollen growth transition in self-incompatible Petunia inflata. Sex Plant Reprod 15:243–253
Messerli MA, Robinson KR (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217:147–157
Montaner C, Floris E, Alvarez JM (2003) Study of pollen cytology and evaluation of pollen viability using in vivo and in vitro test, in borage (Borago officinalis L.). Grana 42:33–37
Mulcahy G, Mulcahy DL (1988) The effect of supplemented media on the growth in vitro of bi- and trinucleate pollen. Plant Sci 55:213–216
Palevitz BA (1993) Relationship between the generative cell and the vegetative nucleus in pollen tubes of Nicotiana tabacum. Sex Plant Reprod 6:1–10
Parton E, Vervaeke I, Delen R, Vandenbussche B, Deroose R, De Proft MP(2002) Viability and storage of bromeliad pollen. Euphytica 125:155–161
Pasonen HL, Kapyla M (1998) Pollen-pollen interactions in Betula pendula in vitro. New Phytol 138:481–487
Pierson ES, Lichtscheidl IK, Derksen J (1990) Structure and behaviour of organelles in living pollen tubes of Lilium longiflorum. J Exp Bot 41:1461–1468
Read SM, Clarke AE, Bacic A (1993a) Requirements for division of the generative nucleus in cultured pollen tubes of Nicotiana. Protoplasma 174:101–115
Read SM, Clarke AE, Bacic A (1993b) Stimulation of growth of cultured Nicotiana tabacum W38 pollen tube by poly(ethylene glycol) and Cu(II) salts. Protoplasma 177:1–14
Rihova L, Hrabetova E, Túpy J (1996) Optimization of conditions for in vitro pollen germination and tube growth in potatoes. Int J Plant Sci 157:561–566
Schreiber DN, Dresselhaus T (2003) In vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Mol Biol Rep 21:31–41
Sharma N, Shivanna KR (1983) Pollen diffusates of Crotalaria retusa and their role in pH regulation. Ann Bot 52:165–170
Shivanna KR (2003) Pollen biology and biotechnology. Science, Enfield
Shivanna KR, Sawhney VK (1995) Polyethylene glycol improves the in vitro growth of Brassica pollen tubes without loss in germination. J Exp Bot 46:1771–1774
Stephenson AG, Travers SE, Mena-Ali JI, Winsor JA (2003) Pollen performance before and during the autotrophic–heterotrophic transition of pollen tube growth. Phil Trans R Soc London 358:1009–1017
Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen-pistil interactions. Annu Rev Genet 38:793–818
Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39:343–353
Taylor LP, Hepler PK (1997) Pollen germination and pollen tube growth. Annu Rev Plant Physiol Plant Mol Biol 48:461–491
Túpy J, Rihova L (1984) Changes and growth effect of pH in pollen tube culture. J Plant Physiol 115:1–10
Vervaeke I, Parton E, Maene L, Deroose R, De Proft MP (2002) Pollen tube growth and fertilization after different in vitro pollination techniques of Aechmea fasciata. Euphytica 124:75–83
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2003) Flower biology of six cultivars of the Bromeliaceae. II. Pollination and fertilization. Selbyana 24:87–94
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2004a) Division of the generative nucleus in cultured pollen tubes of the Bromeliaceae. Plant Cell Tissue Organ Cult 76:17–28
Vervaeke I, Delen R, Wouters J, Deroose R, De Proft MP (2004b) Semi in vivo pollen tube growth of Aechmea fasciata. Plant Cell Tissue Organ Cult 76:67–73
Vervaeke I, Stichelbout L, Londers E, Deroose R, De Proft MP (2005) Influence of arginine, DFMO and polyamines on the division of the generative nucleus in cultures pollen tubes of Aechmea fasciata (Bromeliaceae). Plant Cell Tissue Organ Cult (in press)
Wolukau JN, Zhang S, Xu G, Chen D (2004) The effect of temperature, polyamines and polyamines synthesis inhibitor on in vitro pollen germination and pollen tube growth. Sci Hortic 99:289–299
Acknowledgements
This research is supported by IWT (Flemish Institute to Promote Scientific and Technological Research in the Industry), grant IWT ozm 030507. The authors thank Deroose Plants NV for the plant material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vervaeke, I., Londers, E., Piot, G. et al. The division of the generative nucleus and the formation of callose plugs in pollen tubes of Aechmea fasciata (Bromeliaceae) cultured in vitro. Sex Plant Reprod 18, 9–19 (2005). https://doi.org/10.1007/s00497-005-0243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-005-0243-2