Abstract
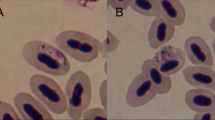
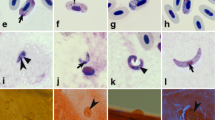
Haemoproteus parasites (Haemosporida, Haemoproteidae) are widespread; some species cause severe diseases in avian hosts. Heavy Haemoproteus infections are often lethal for biting midges (Ceratopogonidae), which transmit avian haemoproteids, but there is no information regarding detrimental effect on other blood-sucking insects. We examined effects of Haemoproteus tartakovskyi (lineage hSISKIN1), Haemoproteus lanii (lineages hRB1and hRBS2) and Haemoproteus balmorali (lineage hCOLL3) on the survival of Ochlerotatus cantans, a widespread Eurasian mosquito. Wild-caught females were infected by allowing them to feed on naturally infected birds with light (0.01 %) and high (3.0–9.6 %) parasitaemia. Mosquitoes fed on uninfected birds were used as controls. Both experimental and control groups were maintained under the same laboratory conditions until 20 days post-exposure (dpe). Dead insects were counted daily and used for parasitological examination and PCR-based testing. No difference was discernible in the survival rate of control mosquitoes and those fed on meal with light parasitaemia. There was a highly significant difference in the survival rate between the control group and all groups fed on meals with high parasitaemia, with the greatest mortality reported 1–3 dpe. For 4 dpe, the percentage of survived control mosquitoes (88 %) was 2.2-, 3.6- and 4-fold greater than that of groups fed on meals with high parasitaemia of H. balmorali, H. tartakovskyi and H. lanii, respectively. Numerous ookinetes were observed in the gut area and adjacent tissues located in the head, thorax and abdomen of infected insects 0.5–1 dpe. The migrating parasites damage organs throughout the entire body of mosquitoes; that is the main reason of mortality. To the end of this study, 46 % of mosquitoes survived in control group, but the survival rates of experimental mosquitoes fed on meals with high parasitaemia were between 2.6- and 5.8-fold lower. This study indicates that widespread Haemoproteus infections are markedly virulent for bird-biting mosquitoes, which rapidly die after feeding on heavily infected blood meals.



Similar content being viewed by others
References
Allison FR, Desser SS, Whitten LK (1978) Further observations on the life cycle and vectors of the haemosporidian Leucocytozoon tawaki and its transmission to the Fiordland crested penguin. N Z J Zool 5:371–374. doi:10.1080/03014223.1978.10428323
Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, Ley SV, Sinden RE (2001) Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasit 116:17–24. doi:10.1016/S0166-6851(01)00299-7
Atkinson CT, Forrester DJ, Greiner EC (1988) Pathogenicity of Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J Parasitol 74:228–239
Barillas-Mury C, Wizel B, Soo Han Y (2000) Mosquito immune responses and malaria transmission: lessons from insect model systems and implications for vertebrate innate immunity. Insect Biochem Mol Biol 30:429–442. doi:10.1016/S0965-1748(00)00018-7
Becker N, Petric D, Zgomba M, Boase C, Dahl C, Lane J, Kaiser A (2003) Mosquitoes and their control. Plenum, New York
Beier JC (1998) Malaria parasite development in mosquitoes. Annu Rev Entomol 43:519–543. doi:10.1146/annurev.ento.43.1.519
Bensch S, Stjenman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Torres-Pinheiro R (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc B 276:1583–1589
Bernotienė R (2012) The fauna and seasonal activity of mosquitoes (Diptera: Culicidae) in the Curonian Spit (Russia, Lithuania). Euro Mosq Bull 30:72–78
Cannell BL, Krasnec KV, Campbell K, Jones HI, Miller RD, Stephens N (2013) The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor). Vet Parasitol 197:74–84. doi:10.1016/j.vetpar.2013.04.025
Desser SS, Yang YJ (1973) Sporogony of Leucocytozoon spp. in mammalophilic simuliids. Can J Zool 51:793
Ejiri H, Sato Y, Sawai R, Sasaki E, Matsumoto R, Ueda M, Higa Y, Tsuda Y, Omori S, Murata K, Yukawa M (2009) Prevalence of avian malaria parasite in mosquitoes collected at a zoological garden in Japan. Parasitol Res 105:629–633. doi:10.1007/s00436-009-1434-9
Ferguson HM, Read AF (2002) Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol 18:256–261. doi:10.1016/S1471-4922(02)02281-X
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell Scientific, Oxford
Glaizot O, Fumagalli L, Iritano K, Lalubin F, Van Rooyen J, Christe P (2012) High prevalence and lineage diversity of avian malaria in wild populations of great tits (Parus major) and mosquitoes (Culex pipiens). PLoS One 7:e34964. doi:10.1371/journal.pone.0034964
Greiner EC, Bennett GF, White EM, Coombs RF (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser (Oxf) 41:95–98
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Ishtiaq F, Guillaumot L, Clegg SM, Phillimore AB, Black RA, Owens IPF, Mundy NI, Sheldon BC (2008) Avian haematozoan parasites and their associations with mosquitoes across Southwest Pacific Islands. Mol Ecol 17:4545–4555. doi:10.1111/j.1365-294X.2008.03935.x
Jahan N, Docherty PT, Billingsley PF, Hurd H (1999) Blood digestion in the mosquito, Anopheles stephensi: the effects of Plasmodium yoelii nigeriensis on midgut enzyme activities. Parasitology 119:535–541. doi:10.1017/S0031182099005090
Kim KS, Tsuda Y, Sasaki T, Kobayashi M, Hirota Y (2009) Mosquito blood-meal analysis for avian malaria study in wild bird communities: laboratory verification and application to Culex sasai (Diptera: Culicidae) collected in Tokyo, Japan. Parasitol Res 105:1351–1357. doi:10.1007/s00436-009-1568-9
Lehane M (2005) The biology of blood-sucking insects, 2nd edn. Cambridge University Press, Cambridge
Marzal A, de Lopes F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
McClure HE, Poonswad P, Greiner EC, Laird M (1978) Haematozoa in the birds of Eastern and Southern Asia. Memorial University of Newfoundland, St. John's
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc Biol Sci 267:2507–2510
Njabo KY, Cornel AJ, Bonneaud C, Toffelmier E, Sehgal RN, Valkiūnas G, Russell AF, Smith TB (2011) Nonspecific patterns of vector, host and avian malaria parasites associations in a central African rainforest. Mol Ecol 20:1049–1061. doi:10.1111/j.1365-294X.2010.04904.x
Palinauskas V, Križanauskienė A, Iezhova TA, Bolshakov CV, Jönsson J, Bensch S, Valkiūnas G (2013) A new method for isolation of purified genomic DNA from haemosporidian parasites inhabiting nucleated red blood cells. Exp Parasitol 133:275–280. doi:10.1016/j.exppara.2012.12.003
Peirce MA (1981) Distribution and host-parasite check-list of the haematozoa of birds in Western Europe. J Nat Hist 15:419–458
Pérez-Tris J, Hellgren O, Križanauskienė A, Waldenström J, Secondi J, Bonneaud C, Fjeldså J, Hasselquist D, Bensch S (2007) Within-host speciation of malaria parasites. PLoS One 2:e235. doi:10.1371/journal.pone.0000235
Santiago-Alarcon D, Palinauskas V, Schaefer HH (2012) Diptera vectors of avian haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Shurulinkov P, Ilieva M (2009) Spatial and temporal differences in the blood parasite fauna of passerine birds during the spring migration in Bulgaria. Parasitol Res 104:1453–1458. doi:10.1007/s00436-009-1349-5
Sinden RE (1998) Gametogenesis and sexual development. In: Sherman IW (ed) Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington, D.C., pp 25–48
Synek P, Munclinger P, Albrecht T, Votýpka J (2013) Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol Res 112:839–845. doi:10.1007/s00436-012-3204-3
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
Valkiūnas G, Iezhova TA (2004) Detrimental effects of Haemoproteus infections on the survival of biting midge Culicoides impunctatus (Diptera: Ceratopogonidae). J Parasitol 90:194–196. doi:10.1645/GE-3206RN
Valkiūnas G, Iezhova TA, Shapoval AP (2003) High prevalence of blood parasites in hawfinch Coccothraustes coccothraustes. J Nat Hist 37:2647–2652. doi:10.1080/002229302100001033221
Valkiūnas G, Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA (2013) Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitol Res 112:2159–2169. doi:10.1007/s00436-013-3375-6
Zehtindjiev P, Ivanova K, Mariaux J, Georgiev BB (2013) First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitol Res 112:3509–3515. doi:10.1007/s00436-013-3533-x
Acknowledgments
The authors are grateful to the staff of the Biological Station ‘Rybachy’ for their assistance in the field. The director of the Biological Station Rybachy, Casimir V. Bolshakov, is acknowledged for generously providing facilities for the experimental research. The experiments described herein comply with the current laws of Lithuania and Russia. This study was funded by the European Social Fund under the Global Grant measure (VPI-3.1.-ŠMM-07-K-01-047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valkiūnas, G., Kazlauskienė, R., Bernotienė, R. et al. Haemoproteus infections (Haemosporida, Haemoproteidae) kill bird-biting mosquitoes. Parasitol Res 113, 1011–1018 (2014). https://doi.org/10.1007/s00436-013-3733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3733-4