Abstract
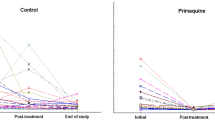
Malarial parasites are supposed to have strong negative fitness consequences for their hosts, but relatively little evidence supports this claim due to the difficulty of experimentally testing this. We experimentally reduced levels of infection with the blood parasite Haemoproteus prognei in its host the house martin Delichon urbica, by randomly treating adults with primaquine or a control treatment. Treated birds had significantly fewer parasites than controls. The primaquine treatment increased clutch size by 18%; hatching was 39% higher and fledging 42% higher. There were no effects of treatment on quality of offspring, measured in terms of tarsus length, body mass, haematocrit or T-cell-mediated immune response. These findings demonstrate that malarial parasites can have dramatic effects on clutch size and other demographic variables, potentially influencing the evolution of clutch size, but also the population dynamics of heavily infected populations of birds.

Similar content being viewed by others
References
Abbas AK, Lichtman AH, Pober JS (1994) Cellular and molecular immunology. Saunder, Philadelphia, Pa.
Allander K (1997) Reproductive investment and parasite susceptibility in the great tit. Funct Ecol 11:358–364
Allander K, Sundberg J (1997) Temporal variation and reliability of blood parasite levels in captive yellowhammer males Emberiza citrinella. J Avian Biol 28:325–330
Applegate JE, Beaudoin RL (1970) Mechanism of spring relapse in avian malaria: effect of gonadotropin and corticosterone. J Wildl Dis 6:443–447
Atkinson C, Van Riper C III (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon and Haemoproteus. In: Loye JE, Zuk M (eds) Bird–parasite interactions. Oxford University Press, Oxford, pp 19–48
Bennett GF, Caines JR, Bishop MA (1988) Influence of blood parasites on the body mass of Passeriform birds. J Wildl Dis 24:339–343
Bennett GF, Peirce MA, Ashford RW (1993) Avian haematozoa: mortality and pathogenicity. J Nat Hist Lond 26:993–1001
Birkhead TR, Fletcher F, Pellatt EJ (1999) Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc R Soc Lond Ser B 266:385–390
Blount JD, Houston DC, Surai PF, Møller AP (2004) Egg-laying capacity is limited by carotenoid pigment availability in wild gulls Larus fuscus. Proc R Soc Lond Ser B 271[Suppl]:S79–S81
Chen M, Shi L, Sullivan D Jr (2001) Haemoproteus and Schitosoma synthesize heme polymers similar to Plasmodium hemozoin and β-hematin. Mol Biochem Parasitol 113:1–8
Chernin E (1952) The relapse phenomenon in Leucocytozoon infections of the domestic duck. Am J Hyg 56:101–118
Christe P, Møller AP, de Lope F (1998) Immunocompetence and nestling survival in the house martin: “the tasty chick hypothesis”. Oikos 83:175–179
Christe P, Møller AP, Saino N, de Lope F (2000) Genetic and environmental components of phenotypic variation in immune response and body size of a colonial bird, the house martin Delichon urbica. Heredity 85:75–83
Combes C (2001) Parasitism. University of Chicago Press, Chicago, Ill.
Dawson RD, Bortolotti GR (2000) Effects of hematozoan parasites on condition and return rates of American kestrels. Auk 117:373–380
González G, Sorci G, de Lope F (1999) Seasonal variation in the relationship between cellular immune response and badge size in male house sparrows (Passer domesticus). Behav Ecol Sociobiol 46:117–122
Goto N, Kodama H, Okada K, Fujimoto Y (1978) Suppression of phytohaemagglutinin skin response in thymectomized chickens. Poult Sci 52:246–250
Hõrak P, Tegelmann L, Ots I, Møller AP (1999) Immune function and survival of great tit nestlings in relation to growth conditions. Oecologia 121:316–322
Hochberg ME, Michalakis Y, de Meeus T (1992) Parasitism as a constraint on the rate of life-history evolution. J Evol Biol 5:491–504
Korpimäki E, Tolonen P, Bennett GF (1995) Blood parasites, sexual selection and reproductive success of European kestrels. Ecoscience 2:335–343
Lehmann T (1993) Ectoparasites: direct impact on host fitness. Parasitol Today 9:8–13
Martin T, Møller AP, Merino S, Clobert J (2001) Does clutch size evolve in response to parasites and immunocompetence? Proc Natl Acad Sci U S A 98:2071–2076
Mayorga P, Deharo E, Landay I, Couarraze G (1997) Preliminary evaluation of primaquine activity on rodent malaria model after transdermal administration. Parasite 4:87–90
Merilä J, Andersson M (1999) Reproductive effort and success are related to hematozoan infection in blue tits. Ecoscience 6:421–428
Merino S, Potti J, Moreno J (1996) Maternal effort mediates the prevalence of trypanosomes in the offspring of a passerine bird. Proc Natl Acad Sci U S A 93:5726–5730
Merino S, Potti J, Fargallo JA (1997) Blood parasites of some passerine birds from Central Spain. J Wildl Dis 33:638–641
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc R Soc Lond Ser B 267:2507–2510
Miller LH, Baruch DR, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415:673–679
Møller AP (1974) A three-year study in colonies of house martins (Delichon urbica) by means of artificial nests. Flora Fauna 80:74–80
Møller AP (1991) Ectoparasite loads affect optimal clutch size in swallows. Funct Ecol 5:351–359
Møller AP (1997) Parasitism and the evolution of host life history. In: Clayton DH, Moore J (eds) Host-parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 105–127
Navarro C, Marzal A, de Lope F, Møller AP (2003) Dynamics of an immune response in house sparrow Passer domesticus in relation to time of day, body condition and blood parasite infection. Oikos 101:291–298
Noble ER, Noble GA (1976) Parasitology. Lea and Febiger, Philadelphia, Pa.
Oppliger A, Christe P, Richner H (1996) Clutch size and malaria resistance. Nature 381:565
Oppliger A, Christe P, Richner H (1997) Clutch size and malarial parasites in female great tits. Behav Ecol 8:148–152
Pajuelo L, de Lope F, da Silva E (1992) Biología de la reproducción del avión común (Delichon urbica) en Badajoz, España. Ardeola 39:15–23
Parmentier HK, Scharma JW, Meijer F, Nieuwland MGB (1993) Cutaneous hypersensitivity responses in chickens divergently selected for antibody responses to sheep red blood cells. Poult Sci 72:1679–1692
Price PV (1980) Evolutionary biology of parasites. Princeton University Press, Princeton, N.J.
Redig PT, Talbot B, Guamera T (1993) Avian malaria. In: Proceedings of the Annual Conference of the Association of Avian Veterinarians. AAV, Lake Worth, Fla., pp 173–181
Richner H, Heeb P (1995) Are clutch and brood size patterns in birds shaped by ectoparasites? Oikos 73:435–441
Roff DA (2001) Life history evolution. Sinauer Associates, Sunderland, Mass.
Saino N, Calza S, Møller AP (1997) Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J Anim Ecol 66:827–836
Saino N, Ferrari R, Romano M, Martinelli R, Møller AP (2003) Experimental manipulation of egg quality affects immunity of barn swallow nestlings. Proc R Soc Lond Ser B 270:2485–2489
Sanz JJ, Arriero E, Moreno J, Merino S (2001) Interactions between hemoparasite status and female age in the primary reproductive output of pied flycatchers. Oecologia 126:339–344
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Smits J, Bortolotti G, Tella J (1999) Simplifying the phytohemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Soler M, Martín-Vivaldi M, Marín JM, Møller AP (1999) Weight lifting and health status in the black wheatear. Behav Ecol 10:281–286
Sundberg J (1995) Parasites, plumage coloration and reproductive success in the yellowhammer, Emberiza citrinella. Oikos 74:331–339
Surai PF (2003) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Wakelin D (1996) Immunity to parasites: how parasitic infections are controlled. Cambridge University Press, Cambridge
Wiehn J, Korpimäki E, Pen I (1999) Haematozoan infections in the Eurasian kestrel: effects of fluctuating food supply and experimental manipulation of paternal effort. Oikos 84:87–98
Acknowledgements
M. Reviriego kindly helped with field work. This study was supported by grants from the Spanish Ministry of Science and Technology BOS 2003-01713 for F. L. and A. M. R., and A. M. R. was supported by a predoctoral grant from Junta de Extremadura (FIC01A043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marzal, A., Lope, F.d., Navarro, C. et al. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142, 541–545 (2005). https://doi.org/10.1007/s00442-004-1757-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1757-2