Abstract
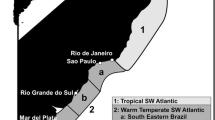
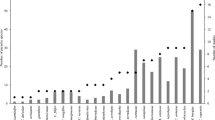
The metazoan parasite fauna and feeding ecology of 165 Sprattus sprattus (L., 1758) was studied from different geographic regions (Baltic Sea, North Sea, English Channel, Bay of Biscay, Mediterranean Sea). A total of 13 metazoan parasite species were identified including six Digenea, one Monogenea, two Cestoda, two Nematoda and two Crustacea. Didymozoidae indet., Lecithocladium excisum and Bomolochidae indet. represent new host records. The parasite species richness differed according to regions and ranged between 3 and 10. The most species-rich parasite fauna was recorded for sprats from the Bay of Biscay (North Atlantic), and the fishes from the Baltic Sea contained the lowest number of parasite species. More closely connected geographical regions, the North Sea, English Channel and Bay of Biscay, showed more similar parasite component communities compared with more distant regions. From the examined stomachs of S. sprattus, a total of 11 different prey items were identified, including Mollusca, Annelida, Crustacea and Tunicata. The highest number of prey organisms belonged to the crustaceans. The variety of prey items in the stomach was reflected by the parasite community differences and parasite species richness from the different regions. The feeding ecology of the fish at the sampled localities was responsible for the observed parasite composition and, secondarily, the zoogeographical distribution of the parasites, questioning the use of the recorded sprat parasites as biological indicators for environmental conditions and change.


Similar content being viewed by others
References
Abollo E, D’Amelio S, Pascual S (2001a) Fitness of the marine parasitic nematode Anisakis simplex s. str. in temperate waters of the NE Atlantic. Dis Aquat Organ 45:131–139
Abollo E, Gestal C, Pascual S (2001b) Anisakis infestation in marine fish and cephalopods from Galician waters: an updated perspective. Parasitol Res 87(6):492–499
Arthur JR (1997) Recent advances in the use of parasites as biological tags for marine fish. In: Flegel TW, MacRae IH (eds) Diseases in Asian aquaculture III. Fish Health Section, Asian Fisheries Society, Manila, pp 141–154
Arthur JR, Albert E (1993) Use of parasites for separating stocks of Greenland halibut (Reinhardtius hippoglossoides) in the Canadian Northwest Atlantic. Can J Fish Aquat Sci 50(10):2175–2181
Bell JJ, Barnes KA (2003) Effect of disturbance on assemblages: an example using Porifera. Biol Bull 205:144–159
Berland B (1998) Biology of Hysterothylacium species. In: Tada I, Kojima S, Tsuji M (eds) Proceedings of the 9th International Congress on Parasitology, Chiba, Japan, pp 373–378
Bush O, Lafferty AD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on his own terms: Margolis et al. revisited. J Parasitol 83:575–583
Cardinale M, Casini M, Arrhenius F (2002) The influence of biotic and abiotic factors on the growth of sprat (Sprattus sprattus) in the Baltic Sea. Aquat Living Res 15:272–281
Cardinale M, Casini M, Arrhenius F, Håkansson N (2003) Diel spatial distribution and feeding activity of herring (Clupea harengus) and sprat (Sprattus sprattus) in the Baltic Sea. Aquat Living Res 16:283–292
Casini M, Cardinale M, Hjelm J (2006) Inter-annual variation in herring, Clupea harengus, and sprat, Sprattus sprattus, condition in the central Baltic Sea: what gives the tune? Oikos 112:638–650
Dailianis T, Limborg M, Hanel R, Bekkevold D, Lagnel J, Magoulas A, Tsigenopoulos CS (2008) Characterization of nine polymorphic microsatellite markers in spratt (Sprattus sprattus L.). Mol Ecol Resour 8:861–863
Dimitrov GI, Bray RA, Gibson DI (1999) A rediscription of Pseudobacciger harengulae (Yamagutti, 1938) (Digenea: Faustulidae) from Sprattus sprattus phalericus (Risso) and Engraulis encrassicholus ponticus Alexandrov off the Bulgarian Black Sea coast, with a review of the genus Pseudobacciger Nahhas & Cable, 1964. Syst Parasitol 43:133–146
Dzikowski R, Paperna I, Diamant A (2003) Use of fish parasite species richness indices in analyzing anthropogenically impacted coastal marine ecosystems. Helgol Mar Res 57:220–227
Fiedler K (1991) Fische: In: Kaestner A. (eds) Lehrbuch der speziellen Zoologie. 2. Teil, Bd. II. Gustav Fischer Verlag Stuttgart: pp 498
Fischer E (1955) Die parasitischen Würmer der wirtschaftlich wichtigsten Ostseefische. PhD thesis, Humboldt-University Berlin: 136 Seiten
Gibson DI, Bray RA (1986) The Hemiuridae (Digenea) of fishes from the north-east Atlantic. Bulletin of the British Museum (Natural History), Zoology Series 51:1–125
Gibson DI, Harris EA, Bray RA, Jepson PD, Kuiken T, Baker JR, Simpson VR (1998) A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J Zool London 244:563–574
Groenewold S (1992) Zur Bedeutung von Kleinfischparasiten im Nordfriesischen Wattenmeer. MSc thesis, Universität Hamburg: 86 Seiten
Groenewold S, Berghahn R, Zander CD (1996) Parasite communities of four fish species in the Wadden Sea and the role of fish discarded by the shrimp fisheries in parasite transmission. Helgoländer Meeresuntersuchungen 50:69–85
Herreras MV, Kaarstad SE, Balbuena JA, Kinze CC, Raga JA (1997) Helminth parasites of digestive tract of the harbour porpoise Phocoena phocoena in Danish waters: a comparative geographical analysis. Dis Aquat Organ 28:163–167
Hyslop EJ (1980) Stomach contents analysis—a review of methods and their application. J Fish Biol 17:411–429
Kelly-Gerreyn BA, Hydes DJ, Jégou AM, Lazure P, Fernand LJ, Puillat I, Garcia-Soto C (2006) Low salinity intrusions in the western English Channel. Continent Shelf Res 26(11):1241–1257
Kleinertz S (2010) Fish parasites as bioindicators: environmental status of coastal marine ecosystems and a grouper mariculture farm in Indonesia. PhD thesis of Natural Sciences, Department 2 (Biology/Chemistry), University of Bremen, 263 pp
Klimpel S, Palm HW (2011) Anisakid nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change? In: Mehlhorn H (ed) Progress in parasitology. Parasitology Research Monographs, vol 2. Springer, Berlin. doi:10.1007/978-3-642-21396-0_11
Klimpel S, Rückert S (2005) Life cycle strategy of Hysterothylatium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol Res 97:141–149
Klimpel S, Palm HW, Seehagen A (2003) Metazoan parasites and feeding behaviour of four small-sized fish species from the central North Sea. Parasitol Res 91:290–297
Klimpel S, Palm HW, Rückert S (2004) The life cycle of Anisakis simplex in the Norwegian Deep (northern North Sea). Parasitol Res 94:1–9
Klimpel S, Kleinertz S, Hanel R, Rückert S (2007) Genetic variability in Hysterothylacium, a raphidascarid nematode isolated from sprat (Sprattus sprattus) of different geographical areas of the northeastern Atlantic. Parasitol Res 101:1425–1430
Klimpel S, Kleinertz S, Palm HW (2008) Distribution of parasites from red mullets (Mullus surmuletus L., Mullidae) in the North Sea and Mediterranean Sea. Bull Fish Biol 10:25–38
Klimpel S, Busch MW, Kellermanns E, Kleinertz S, Palm HW (2009) Metazoan deep-sea fish parasites. Acta Biologica Benrodis, Supplement 11, Natur & Wissen Verlag, Solingen: 384 Seiten
Køie M (1979) On the morphology and life-history of Derogenes varicus (Müller, 1784) Looss, 1901 (Trematoda, Hemiuridae). Zeitschrift für Parasitenkunde (Parasitol Res) 59:67–78
Køie M (1990) On the morphology and life-history of Hemiurus luehei Odhner, 1905 (Digenea: Hemiuridae). J Helminthol 64:193–202
Køie M (1992) Life cycle and structure of the fish digenean Brachyphallus crenatus (Hemiuridae). J Parasitol 78:338–343
Køie M (1993) Aspects of the life-cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (nematoda, Ascaridoidea, Anisakidea). Can J Zool 71:1289–1296
Køie M, Lester RJG (1985) Larval didymozoids (Trematoda) in fishes from Moreton Bay, Australia. Proc Helminthol Soc Wash 52:196–203
Lazure P, Jégou AM, Kerdreux M (2006) Analysis of salinity measurements near islands on the French continental shelf of the Bay of Biscay. In: Morán XAG, Rodriguez JM, Petigas P (eds) Oceanography of the Bay of Biscay. Scientia Marina, 70S1, Barcelona (Spain), pp 7–14. ISSN: 0214–8358
Limborg MT, Pedersen JS, Hemmer-Hansen J, Tomkiewicz J, Bekkevold D (2009) Genetic population structure of European sprat Sprattus sprattus: differentiation across a steep environmental gradient in a small pelagic fish. Marine Ecol Progr Ser 379:213–224
Love MS, Moser M (1983) A checklist of parasites of Californian, Oregon and Washington marine and estuarine fishes. NOAA Technical Report NMFS SSRF 777:1–577
MacKenzie K (1983) Parasites as biological tags in fish population studies. Adv Appl Biol 7:251–331
MacKenzie K (1985) The use of parasites as biological tags in population studies of herring (Clupea harengus L.) in the North Sea and to north and west of Scotland. J Intl Council Explor Sea 42:33–64
MacKenzie K (1987) Long-term changes in the prevalence of two helminth parasites (Cestoda: Trypanorhyncha) infecting marine fish. J Fish Biol 31:83–87
MacKenzie K (1990) Cestode parasites as biological tags for mackerel (Scomber scombrus L.) in the northeast Atlantic. J Intl Council Explor Sea 46:155–166
Magurran AE (1988) Ecological diversity and its measurement. Croom Helm, London
Marcogliese DJ (1995) The role of zooplankton in the transmission of helminth parasites to fish. Rev Fish Biol Fisheries 5:336–371
Marcogliese DJ (1996) Larval parasitic nematodes infecting marine crustaceans in eastern Canada. 3. Hysterothylacium aduncum. J Helminthol Soc Washington 63(1):12–18
Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124:83–99
Margolis L, Arthur JR (1979) Synopsis of the parasites of fishes of Canada. Bull Fisheries Res Board Canada 199:1–269
Möllmann C, Kornilovs G, Fetter M, Köster FW (2004) Feeding ecology of central Baltic Sea herring and sprat. J Fish Biol 65:1563–1581
Nikolaeva VM (1965) On the developmental cycle of trematodes belonging to the family Didymozoidae. Zlogicheski Zhurnal 44:1317–1327
Palm HW (2011) Fish parasites as biological indicators in a changing world: can we monitor environmental impact and climate change? In: Mehlhorn H (ed) Progress in parasitology. Parasitology Research Monographs, vol 2, Springer, Berlin. doi:10.1007/978-3-642-21396-0_12
Palm HW, Dobberstein RC (1999) Occurrence of trichodinid ciliates (Peritricha: Urceolariidae) in the Kiel Fjord, Baltic Sea, and its possible use as a biological indicator. Parasitol Res 85:726–732
Palm HW, Rückert S (2009) A new approach to visualize fish and ecosystem health by using parasites. Parasitol Res 105:539–553
Palm HW, Klimpel S, Bucher C (1999) Checklist of metazoan fish parasites of German coastal waters. Berichte aus dem Institut für Meereskunde 307:1–148
Palm HW, Kleinertz S, Rückert S (2011) Parasite diversity as an indicator of environmental change?—An example from tropical grouper (Epinephelus fuscoguttatus) mariculture in Indonesia. Parasitology 138:1–11. doi:10.1017/S0031182011000011
Pinkas L, Oliphant MD, Iverson ILK (1971) Food habits of albacore, bluefin tuna and bonito in Californian waters. California Fish and Game 152:1–105
Reimer LW (1978) Parasiten von Sprotten III. Wissenschaftliche Konferenz zu Fragen der Physiologie und Biologie von Nutzfischen, 7.-8.9.1978 in Rostock, pp 147–152
Rheinheimer G (ed) (1995) Meereskunde der Ostsee. 2. Auflage, Springer, 338 pp
Riemann F (1988) Nematoda. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, pp 239–301
Rother K (1993) Der Mittelmeerraum. Teubner Studienbücher der Geographie,183 pp
Sasal P, Mouillot D, Fichez R, Chifflet S, Kulbicki M (2007) The use of fish parasites as biological indicators of anthropogenic influences in coral-reef lagoons: a case study of Apogonidae parasites in New-Caledonia. Mar Pollut Bull 54:1697–1706
Strømnes E, Andersen K (2000) “Spring rise” of whaleworm (Anisakis simplex; Nematoda, Ascaridoidea) third stage larvae in some fish species from Norwegian waters. Parasitol Res 86:619–624
Sures B (2008) Host–parasite interactions in polluted environments. J Fish Biol 73:2133–2142
Tolonen A, Karlsbakk E (2003) The parasite fauna of the Norwegian spring spawning herring (Clupea harengus L.). ICES Journal of Marine Science 60:77–84
Vidal-Martínez VM, Pech D, Sures B, Purucker ST, Poulin R (2010) Can parasites really reveal environmental impact? Trends Parasitol 26(1):44–51
Williams HH, MacKenzie K, McCarthy AM (1992) Parasites as biological indicators of the population biology, migrations, diet and phylogenetics of fish. Rev Fish Biol Fisheries 2:144–176
Acknowledgements
We are thankful to the scientific staff and crew of the involved research vessels for their assistance during sample collection. We would like to thank H. Mehlhorn who supported parts of the study at Heinrich-Heine-University, Düsseldorf, Institute of Zoomorphology, Cell Biology and Parasitology, and all other members involved at this institute. We would like to thank Sebastian Ferse (Leibniz ZMT Bremen, Germany) for providing help with Sigma Plot.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleinertz, S., Klimpel, S. & Palm, H.W. Parasite communities and feeding ecology of the European sprat (Sprattus sprattus L.) over its range of distribution. Parasitol Res 110, 1147–1157 (2012). https://doi.org/10.1007/s00436-011-2605-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2605-z