Abstract
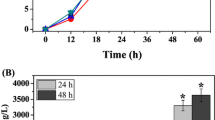
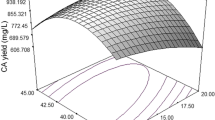
The nutritional requirements for phenazine-1-carboxylic acid (PCA) production using Pseudomonas sp. M18G, a gacA chromosomal-inactivated mutant of the strain M18, with a high PCA yield, were optimized statistically in shake flask experiments. Based on a single-factor experiment design, we implemented the two-level Plackett–Burman (PB) design with 11 variables to screen medium components that significantly influence PCA production. Soybean meal, glucose, soy peptone, and ethanol were identified as the most important significant factors (P < 0.05). Response surface methodology based on the Center Composite Design (CCD) was applied to determine these factors’ optimal levels and their mutual interactions between components for PCA production. The predicted results showed that 1.89 g l−1 of PCA production was obtained after a 60-h fermentation period, with optimal concentrations of soybean meal powder (33.4 g l−1), glucose (12.7 g l−1), soy peptone (10.9 g l−1), and ethanol (13.8 ml l−1) in the flask fermentations. The validity of the model developed was verified, and the optimum medium led to a maximum PCA concentration of 2.0 g l−1, a nearly threefold increase compared to that in the basal medium. Furthermore, the experiment was scaled up in the 10 l fermentor and 2 g l−1 PCA productions were achieved in 48 h based on optimization mediums which further verified the practicability of this optimum strategy.



Similar content being viewed by others
References
Abdel-Fattah YR, Saeed HM, Gohar YM, El-Baz MA (2005) Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem 40:1707–1714
Adinarayana K, Ellaiah P (2002) Response surface optimization of the critical medium components for the production of alkaline protease by a newly isolated Bacillus sp. J Pharm Pharmacol Sci 5:281–287
Chin-A-Woeng TFC et al (1998) Biocontrol by phenazine-1-carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Mol Plant-Microb Interact 11:1069–1077
Chin-A-Woeng TFC, Thomas-Oates JE, Lugtenberg BJ, Bloemberg GV (2001) Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp. strains.. Mol Plant Microbe Interact 14:1006–1015
Chin-A-Woeng TFC, van den Broek D, Lugtenberg BJJ, Bloemberg GV (2005) The Pseudomonas chlororaphis PCL1391 sigma regulator psrA represses the production of the antifungal metabolite phenazine-1-carboxamide. Mol Plant-Microb Interact 18:244–253
Delaney SM, Mavrodi DV, Bonsall RF, Thomashow LS (2001) phzO, a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens 30–84. J Bacteriol 183:318–327
Duffy BK, Defago G (1999) Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol 65:2429–2438
Ge Y, Huang X, Wang S, Zhang X, Xu Y (2004) Phenazine-1-carboxylic acid is negatively regulated and pyoluteorin positively regulated by gacA in Pseudomonas sp. M18. FEMS Microbiol Lett 237:41–47
Gheshlaghi R, Scharer JM, Moo-Young M, Douglas PL (2005) Medium optimization for hen egg white lysozyme production by recombinant Aspergillus niger using statistical methods. Biotechnol Bioeng 90:754–760
Hamdan H, Weller DM, Thomashow LS (1991) Relative importance of fluorescent siderophores and other factors in biological control of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2–79 and M4–80R. Appl Environ Microbiol 57:3270–3277
Himabindu M, Ravichandra P, Vishalakshi K, Jetty A (2006) Optimization of critical medium components for the maximal production of gentamicin by Micromonospora echinospora ATCC 15838 using response surface methodology. Appl Biochem Biotechnol 134:143–154
Hu HB, Yu QX, Feng C, Xue HZ, Hur BK (2005) Isolation and characterization of a new fluorescent Pseudomonas strain that produces both phenazine 1-carboxylic acid and pyoluteorin. J Microbiol Biotechnol 15:86–90
Kennedy M, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotech 23:456–475
Khambhaty Y, Mody K, Jha B, Gohel V (2007) Statistical optimization of medium components for kappa-carrageenase production by Pseudomonas elongata. Enzyme Microb Technol 40:813–822
Lai LST, Pan CC, Tzeng BK (2003) The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochem 38:1317–1326
Lee JY, Moon SS, Hwang BK (2003) Isolation and in vitro and in vivo activity against Phytophthora capsici and Colletotrichum orbiculare of phenazine-1-carboxylic acid from Pseudomonas aeruginosa strain GC-B26. Pest Manag Sci 59:872–882
Mavrodi DV, Ksenzenko VN, Bonsall RF, Cook RJ, Boronin AM, Thomashow LS (1998) A seven-gene locus for synthesis of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2–79. J Bacteriol 180:2541–2548
Mavrodi DV, Bleimling N, Thomashow LS, Blankenfeldt W (2004) The purification, crystallization and preliminary structural characterization of PhzF, a key enzyme in the phenazine-biosynthesis pathway from Pseudomonas fluorescens 2–79. Acta Crystallogr D Biol Crystallogr 60:184–186
Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS 3rd (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616–2624
Mizumoto S, Shoda M (2007) Medium optimization of antifungal lipopeptide, iturin A, production by Bacillus subtilis in solid-state fermentation by response surface methodology. Appl Microbiol Biotechnol 76:101–108
Parsons JF, Song F, Parsons L, Calabrese K, Eisenstein E, Ladner JE (2004) Structure and function of the phenazine biosynthesis protein PhzF from Pseudomonas fluorescens 2–79. Biochem 43:12427–12435
Pierson LS 3rd, Thomashow LS (1992) Cloning and heterologous expression of the phenazine biosynthetic locus from Pseudomonas aureofaciens 30–84. Mol Plant Microbe Interact 5:330–339
Raaijmakers JM, Weller DM, Thomashow LS (1997) Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl Environ Microbiol 63:881–887
Shtark O, Shaposhnikov AI, Kravchenko LV (2003) The production of antifungal metabolites by Pseudomonas chlororaphis grown on different nutrient sources. Mikrobiologiia 72:645–650
Siddiqui IA, Shaukat SS (2004) Liquid culture carbon, nitrogen and inorganic phosphate source regulate nematicidal activity by fluorescent pseudomonads in vitro. Lett Appl Microbiol 38:185–190
Slininger PJ, Jackson MA (1992) Nutritional factors regulating growth and accumulation of phenazine-1-carboxylic acid by Pseudomonas fluorescens 2–79. Appl Microbiol Biotechnol 37:388–392
Slininger PJ, Shea-Wilbur MA (1995) Liquid-culture pH, temperature, and carbon (not nitrogen) source regulate phenazine productivity of the take-all biocontrol agent Pseudomonas fluorescens 2–79. Appl Microbiol Biotechnol 43:794–800
Tari C, Genckal H, Tokatli F (2006) Optimization of a growth medium using a statistical approach for the production of an alkaline protease from a newly isolated Bacillus sp L21. Process Biochem 41:659–665
Timms-Wilson TM et al (2000) Chromosomal insertion of phenazine-1-carboxylic acid biosynthetic pathway enhances efficacy of damping-off disease control by Pseudomonas fluorescens. Mol Plant Microbe Interact 13:1293–1300
Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol 170:3499–3508
Thomashow LS, Weller DM, Bonsall RF, Pierson LS (1990) Production of the antibiotic phenazine-1-carboxylic acid by fluorescent pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol 56:908–912
van den Broek D, Chin-A-Woeng TFC, Eijkemans K, Mulders IHM, Bloemberg GV, Lugtenberg BJJ (2003) Biocontrol traits of Pseudomonas spp. are regulated by phase variation. Mol Plant-Microbe Interact 16:1003–1012
van Rij ET, Wesselink M, Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2004) Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Mol Plant-Microbe Interact 17:557–566
Vohra A, Satyanarayana T (2002) Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process Biochem 37:999–1004
Wei WL, Zheng ZH, Liu YY, Zhu XS (1998) Optimizing the culture conditions for higher inulinase production by Kluyveromyces sp. Y-85 and scaling-up fermentation. J Ferment Bioeng 86:395–399
Xiao ZJ, Liu PH, Qin JY, Xu P (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74:61–68
Yuan Z, Cang S, Matsufuji M, Nakata K, Nagamatsu Y, Yoshimoto A (1998) High production of pyoluteorin and 2,4-diacetylphloroglucinol by Pseudomonas fluorescens S272 grown on ethanol as a sole carbon source. J Ferment Bioeng 86:559–563
Acknowledgements
This study was supported by the 863 Programs of China (No. 2006AA10A209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Jiang, H., Xu, Y. et al. Optimization of nutrient components for enhanced phenazine-1-carboxylic acid production by gacA-inactivated Pseudomonas sp. M18G using response surface method. Appl Microbiol Biotechnol 77, 1207–1217 (2008). https://doi.org/10.1007/s00253-007-1213-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1213-4