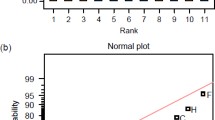
Abstract
Strain 2-79 is a biocontrol agent against take-all, an important disease of wheat caused byGaeumannomyces graminis var.tritici. In the rhizosphere, it produces the antibiotic phenazine 1-carboxylic acid (PCA) as the primary means of disease suppression. One barrier to commercial use of phenazine-producing pseudomonads, like strain 2–79, is the lack of liquid-culture technology for mass production. For instance, there is little published research concerning the impact of liquid-culture secondary metabolism on the biocontrol qulaities of the cell harvest, i.e., efficacy, phytotoxicity, and storage survival. Yet it is important to know whether the fermentation process should be designed to enhance or eliminate secondary metabolite accumulation. To enable future exploration of this issue, we identified liquid-culture parameters that could be manipulated to control the phenazine productivity of strain 2–79. Our results indicated that PCA accumulation was very sensitive to the culture pH and temperature. It was possible to produce large cell populations with either high or low phenazine productivity by choosing to control culture pH at 7 and 8 respectively. Although high cell accumulations were achieved over the broad 25–34°C range studied, high, moderate, or low PCA productivities were observed at 25–27°C, 29–32.5°C, or 34°C respectively. When pH was controlled at 7, specific PCA productions at 25°C could be modulated by the choice of carbon source supplied. PCA accumulation per unit biomass reached 0.31 g/g on glucose, 0.16 g/g on glycerol and xylose, and only 0.09 g/g on fructose. Although the nitrogen source was also tested as a variable, it had little influence on culture PCA productivity under controlled pH.
Similar content being viewed by others
References
Bothast RJ, Schisler DA, Jackson MA, VanCauwenberge JE, Slininger PJ (1993) Use of pregelatinized starch and other polysaccharides for improved storage and efficacy of biocontrol agents. In: Berger PD, Devisetty BN, Hall FR (eds) Pesticide formulations and application systems, vol 13. ASTM, Philadelphia, Pennsylvania, pp 45–56
Brisbane PG, Janik LJ, Tate ME, Warren RO (1987) Revised structure for the phenazine antibiotic fromPseudomonas fluorescens 2–79 (NRRL B-15132). Antimicrob Agents Chemother 31:1967–1971
Bull CT, Weller DM, Thomashow LS (1991) Relationship between root colonization and suppression ofGaeumannomyces graminis var.tritici byPseudomonas fluorescens strain 2–79. Phytopathology 81:954–959
Cook RJ, Veseth RJ (1991) Wheat health managemnet. APS, St Paul, Minn
Gurusiddaiah S, Weller DM, Sarkar A, Cook RJ (1986) Characterization of an antibiotic produced by a strain ofPseudomonas fluorescens inhibitory toGaeumannomyces graminis var.tritici andPythium spp. Antimicrob Agents Chemother 29:488–495
Haynes WC, Stodola FH, Locke JM, Pridham TG, Conway HF, Sohns VE, Jackson RW (1956)Pseudomonas aureofaciens Kluyver and phenazine alpha-carboxylic acid, its characteristic pigment. J Bacteriol 72:412–417
Korth H, Taraz K, Benoni H (1990) Isolation ofPseudomonas fluorescens producing phenazine derivatives exclusively under strains conditions of iron deficiency. Zentralbl Bakteriol 274:433–435
Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS III (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616–2624
Messenger AJM, Turner JM (1983) Effect of growth conditions on phenazine production byPseudomonas phenazinium. J Gen Microbiol 129:1013–1018
Ownley BH, Weller DM, Thomashow LS (1992) Influence of in situ and in vitro pH on suppression ofGaeumannomyces graminis var.tritici byPseudomonas fluorescens 2–79. Phytopathology 82:178–184
Pierson LS, Thomashow LS (1992) Cloning and heterologous expression of the phenazine biosynthetic locus fromPseudomonas aureofaciens 30–84. Mol Plant Microbe Interact 5:330–339
Slininger PJ, Jackson MA (1992) Nutritional factors regulating growth and accumulation of phenazine 1-carboxylic acid byPseudomonas fluorescens 2–79. Appl. Microbiol Biotechnol 37:388–392
Slininger PJ, Schisler DA, Bothast RJ (1994) Two-dimensional liquid culture focusing: a method of selecting commercially promising microbial isolates with demonstrated biological control capability. In: Ryder MH, Stephens PM, Bowen GD (eds) Improving plant productivity with rhizosphere bacteria. CSIRO Division of Soils, Glen Osmond, South Australia, pp 29–32
Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic fromPseudomonas fluorescens in biological control ofGaeumannomyces graminis var.tritici. J Bacteriol 170:3499–3508
Thomashow LS, Weller DM (1990) Role of antibiotics and siderophores in biocontrol of take-all disease of wheat. Plant Soil 129:93–99
Thomashow LS, Weller DM, Bonsall RF, Pierson LS (1990) Production of the antibiotic phenazine 1-carboxylic acid by fluorescentPseudomonas species in the rhizophere of wheat. Appl Environ Microbiol 56:908–912
Toohey JI, Nelson CD, Krotkov G (1965a) Isolation and identification of two phenazines from a strain ofPseudomonas aureofaciens. Can J Bot 43:1055–1062
Toohey JI, Nelson CD, Krotkov G (1965b) Toxicity of phenazine carboxylic acids to some bacteria, algae, higher plants, and animals. Can J Bot 43:1151–1155
Vidaver AK (1967) Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol 15:1523–1524
Weller DM (1988) Biological control of soilborne pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
Weller DM, Cook RJ (1983) Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463–469
Weller DM, Cook RJ (1984) Method for screening bacteria and application thereof for field control of diseases caused byGaeumannomyces graminis. US patent 4 456 684
Zabriskie DW, Arminger WB, Phillips DH, Albano PA (1980)Trader's guide to fermentation media formulation. Trader'd Protein, Memphis, Tenn, p 58
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slininger, P.J., Shea-Wilbur, M.A. Liquid-culture pH, temperature, and carbon (not nitrogen) source regulate phenazine productivity of the take-all biocontrol agentPseudomonas fluorescens 2-79. Appl Microbiol Biotechnol 43, 794–800 (1995). https://doi.org/10.1007/BF02431910
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02431910