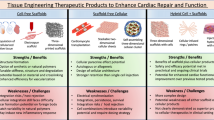
Abstract
Tissue-engineered cardiac constructs are a high potential therapy for treating myocardial infarction. These therapies have the ability to regenerate or recreate functional myocardium following the infarction, restoring some of the lost function of the heart and thereby preventing congestive heart failure. Three key factors to consider when developing engineered myocardial tissue include the cell source, the choice of scaffold, and the use of biomimetic culture conditions. This review details the various biomaterials and scaffold types that have been used to generate engineered myocardial tissues as well as a number of different methods used for the fabrication and culture of these constructs. Specific bioreactor design considerations for creating myocardial tissue equivalents in vitro, such as oxygen and nutrient delivery as well as physical stimulation, are also discussed. Lastly, a brief overview of some of the in vivo studies that have been conducted to date and their assessment of the functional benefit in repairing the injured heart with engineered myocardial tissue is provided.

Similar content being viewed by others
Abbreviations
- MI:
-
Myocardial infarction
- ECM:
-
Extracellular matrix
- FDA:
-
US Food and Drug Administration
- SIS:
-
Small intestinal submucosa
- UBM:
-
Urinary bladder matrix
- GAGs:
-
Glycosaminoglycans
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PNIPAAm:
-
Poly(N-isopropylacrylamide)
- PTMCLA:
-
Poly(trimethylene carbonate-co-lactide)
- VEGF:
-
Vascular endothelial growth factor
- PEG:
-
Polyethylene glycol
- FAK:
-
Focal adhesion Kinase
- RhoA:
-
Ras homolog gene family, member A
- AVL:
-
Arteriovenous blood vessel loop
- FS:
-
Fractional shortening
- LVEF:
-
Left ventricular ejection fraction
- EHTs:
-
Engineered heart tissue
- LVEDD:
-
Left ventricular end-diastolic dimension
- LVEDV:
-
Left ventricular end-diastolic volume
- bFGF:
-
Basic fibroblast growth factor
- CPCs:
-
Cardiac progenitor cells
- IGF-1:
-
Insulin-like growth factor 1
References
Minino, A. M., Xu, J., & Kochanek, K. D. (2008). Deaths: Preliminary data for 2008. National Vital Statistics Reports, vol. 59, p. 31.
World Health Organization (2008). The top ten causes of death, 2008.
Beltrami, A. P., Urbanek, K., Kajstrua, J., Yan, S.-M., Finato, N., Bussani, R., et al. (2001). Evidence that human cardiac myocytes divide after myocardial infarction. The New England Journal of Medicine, 344(23), 1750–1757.
Pfeffer, M. A., & Braunwald, E. (1990). Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation, 81(4), 1161–1172.
Gaudron, P., Eilles, C., Kugler, I., & Ertl, G. (1993). Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation, 87(3), 755–763.
Segers, V. F. M., & Lee, R. T. (2010). Protein therapeutics for cardiac regeneration after myocardial infarction. Journal of Cardiovascular Translational Research, 3(5), 469–477.
Boyle, A. (2009). Current status of cardiac transplantation and mechanical circulatory support. Current Heart Failure Reports, 6(1), 28–33.
Jeevanandam, V., Furukawa, S., Prendergast, T. W., Todd, B. A., Eisen, H. J., & McClurken, J. B. (1996). Standard criteria for an acceptable donor heart are restricting heart transplantation. The Annals of Thoracic Surgery, 62(5), 1268–1275.
Lindenfeld, J., Miller, G. G., Shakar, S. F., Zolty, R., Lowes, B. D., Wolfel, E. E., et al. (2004). Drug therapy in the heart transplant recipient: Part I: Cardiac rejection and immunosuppressive drugs. Circulation, 110(24), 3734–3740.
Laflamme, M. A., Zbinden, S., Epstein, S. E., & Murry, C. E. (2007). Cell-based therapy for myocardial ischemia and infarction: Pathophysiological mechanisms. Annual Review of Pathology, 2, 307–339.
Beitnes, J., Hopp, E., Lunde, K., Solheim, S., Arnesen, H., Brinchmann, J., et al. (2009). Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: The ASTAMI randomised, controlled study. Heart, 95(24), 1983–1989.
Schächinger, V., Assmus, B., Erbs, S., Elsässer, A., Haberbosch, W., Hambrecht, R., et al. (2009). Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: Insights from the Reinfusion of Enriched Progenitor Cells and Infarct Remodelling in Acute Myocardial Infarction (REPAIR-AMI) trial. European Journal of Heart Failure, 11(10), 973–979.
D’Alessandro, D. A., & Michler, R. E. (2010). Current and future status of stem cell therapy in heart failure. Curr Treat Options Cardiovasc Med, 12(6), 614–627.
Meyer, G. P., Wollert, K. C., Lotz, J., Steffens, J., Lippolt, P., Fichtner, S., et al. (2006). Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (Bone Marrow Transfer to Enhance ST-elevation Infarct Regeneration) trial. Circulation, 113(10), 1287–1294.
Terrovitis, J. V., Smith, R. R., & Marbán, E. (2010). Assessment and optimization of cell engraftment after transplantation into the heart. Circulation Research, 106(3), 479–494.
Hattori, F., & Fukuda, K. (2010). Strategies for ensuring that regenerative cardiomyocytes function properly and in cooperation with the host myocardium. Experimental & Molecular Medicine, 42(3), 155.
Choi, Y. S., Dusting, G. J., Stubbs, S., Arunothayaraj, S., Han, X. L., Collas, P., et al. (2010). Differentiation of human adipose-derived stem cells into beating cardiomyocytes. Journal of Cellular and Molecular Medicine, 14(4), 878–889.
Coppen, S. R., Fukushima, S., Shintani, Y., Takahashi, K., Varela-Carver, A., Salem, H., et al. (2008). A factor underlying late-phase arrhythmogenicity after cell therapy to the heart: Global downregulation of connexin43 in the host myocardium after skeletal myoblast transplantation. Circulation, 118(14 Suppl), S138–S144.
Zhang, J., Ding, L., Zhao, Y., Sun, W., Chen, B., Lin, H., et al. (2009). Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation, 119(13), 1776–1784.
Christman, K. L., Fok, H. H., Sievers, R. E., Fang, Q., & Lee, R. J. (2004). Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Engineering, 10(3–4), 403–409.
Padin-Iruegas, M. E., Misao, Y., Davis, M. E., Segers, V. F. M., Esposito, G., Tokunou, T., et al. (2009). Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation, 120(10), 876–887.
Kreutziger, K. L., & Murry, C. E. (2011). Engineered human cardiac tissue. Pediatric Cardiology, 32(3), 334–341.
Martinez, E. C., & Kofidis, T. (2009). Myocardial tissue engineering: The quest for the ideal myocardial substitute. Expert Review of Cardiovascular Therapy, 7(8), 921–928.
St. John Sutton, M. G., & Sharpe, N. (2000). Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation, 101, 2981–2988.
Landa, N., Miller, L., Feinberg, M. S., Holbova, R., Shachar, M., Freeman, I., et al. (2008). Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation, 117(11), 1388–1396.
O’Blenes, S. B., Li, A. W., Chen, R., Arora, R. C., & Horackova, M. (2010). Engraftment is optimal when myoblasts are transplanted early: The role of hepatocyte growth factor. The Annals of Thoracic Surgery, 89(3), 829–835.
Hu, X., Wang, J., Chen, J., Luo, R., He, A., Xie, X., et al. (2007). Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. European Journal of Cardio-Thoracic Surgery, 31(3), 438–443.
Sakamoto, T., Kojima, S., Ogawa, H., Shimomura, H., Kimura, K., Ogata, Y., et al. (2006). Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. The American Journal of Cardiology, 97(8), 1165–1171.
Martin, I., Wendt, D., & Heberer, M. (2004). The role of bioreactors in tissue engineering. Trends in Biotechnology, 22(2), 80–86.
Vunjak-Novakovic, G., Tandon, N., Godier, A., Maidhof, R., Marsano, A., Martens, T. P., et al. (2010). Challenges in cardiac tissue engineering. Tissue Engineering. Part B, Reviews, 16(2), 169–187.
Jawad, H., Ali, N. N., Lyon, A. R., Chen, Q. Z., Harding, S. E., & Boccaccini, A. R. (2007). Myocardial tissue engineering: A review. Journal of Tissue Engineering and Regenerative Medicine, 1, 327–342.
Matsushita, T., Oyamada, M., Fujimoto, K., Yasuda, Y., Masuda, S., Wada, Y., et al. (1999). Remodeling of cell–cell and cell–extracellular matrix interactions at the border zone of rat myocardial infarcts. Circulation Research, 85(11), 1046–1055.
Lokmic, Z., Stillaert, F., Morrison, W. A., Thompson, E. W., & Mitchell, G. M. (2007). An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. The FASEB Journal, 21(2), 511–522.
Tee, R., Lokmic, Z., Morrison, W. A., & Dilley, R. J. (2010). Strategies in cardiac tissue engineering. ANZ Journal of Surgery, 80(10), 683–693.
Zimmermann, W.-H. (2001). Tissue engineering of a differentiated cardiac muscle construct. Circulation Research, 90(2), 223–230.
Zimmermann, W.-H., Melnychenko, I., Wasmeier, G., Didié, M., Naito, H., Nixdorff, U., et al. (2006). Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine, 12(4), 452–458.
Black, L. D., Meyers, J. D., Weinbaum, J. S., Shvelidze, Y. A., & Tranquillo, R. T. (2009). Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Engineering. Part A, 15(10), 3099–3108.
Huang, C.-C., Liao, C.-K., Yang, M.-J., Chen, C.-H., Hwang, S.-M., Hung, Y.-W., et al. (2010). A strategy for fabrication of a three-dimensional tissue construct containing uniformly distributed embryoid body-derived cells as a cardiac patch. Biomaterials, 31(24), 6218–6227.
Wei, H.-J., Chen, C.-H., Lee, W.-Y., Chiu, I., Hwang, S.-M., Lin, W.-W., et al. (2008). Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials, 29(26), 3547–3556.
Colazzo, F., Sarathchandra, P., Smolenski, R. T., Chester, A. H., Tseng, Y.-T., Czernuszka, J. T., et al. (2011). Extracellular matrix production by adipose-derived stem cells: Implications for heart valve tissue engineering. Biomaterials, 32(1), 119–127.
Garbade, J., Schubert, A., Rastan, A. J., Lenz, D., Walther, T., Gummert, J. F., et al. (2005). Fusion of bone marrow-derived stem cells with cardiomyocytes in a heterologous in vitro model. European Journal of Cardio-Thoracic Surgery, 28(5), 685–691.
Jacot, J. G., Martin, J. C., & Hunt, D. L. (2010). Mechanobiology of cardiomyocyte development. Journal of Biomechanics, 43(1), 93–98.
Cavalcanti-Adam, E., Tomakidi, P., Bezler, M., & Spatz, J. (2005). Geometric organization of the extracellular matrix in the control of integrin-mediated adhesion and cell function in osteoblasts. Progress in Orthodontics, 6(2), 232–237.
Li, T.-S., Komota, T., Ohshima, M., Qin, S.-L., Kubo, M., Ueda, K., et al. (2008). TGF-beta induces the differentiation of bone marrow stem cells into immature cardiomyocytes. Biochemical and Biophysical Research Communications, 366(4), 1074–1080.
Bouten, C. V. C., Dankers, P. Y. W., Driessen-Mol, A., Pedron, S., Brizard, A. M. A., & Baaijens, F. P. T. (2011). Substrates for cardiovascular tissue engineering. Advanced Drug Delivery Reviews, 63(4–5), 221–241.
Schwartz, M. A., & DeSimone, D. W. (2008). Cell adhesion receptors in mechanotransduction. Current Opinion in Cell Biology, 20(5), 551–556.
Yoneno, K., Ohno, S., Tanimoto, K., Honda, K., Tanaka, N., Doi, T., et al. (2005). Multidifferentiation potential of mesenchymal stem cells in three-dimensional collagen gel cultures. Journal of Biomedical Materials Research. Part A, 75(3), 733–741.
Radisic, M., Park, H., Shing, H., Consi, T., Schoen, F. J., Langer, R., et al. (2004). Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America, 101(52), 18129–18134.
Kutschka, I., Chen, I. Y., Kofidis, T., Arai, T., von Degenfeld, G., Sheikh, A. Y., et al. (2006). Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation, 114(1 Suppl), I167–I173.
Bauman, L. (2004). Cosmoderm/cosmoplast (human bioengineered collagen) for the aging face. Facial Plastic Surgery, 20(2), 125–128.
Geiger, M., Li, R. H., & Friess, W. (2003). Collagen sponges for bone regeneration with rhBMP-2. Advanced Drug Delivery Reviews, 55(12), 1613–1629.
Kleinman, H. K., & Martin, G. R. (2005). Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology, 15(5), 378–386.
Kofidis, T., de Bruin, J. L., Hoyt, G., Lebl, D. R., Tanaka, M., Yamane, T., et al. (2004). Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. The Journal of Thoracic and Cardiovascular Surgery, 128(4), 571–578.
Williams, C., Johnson, S. L., Robinson, P. S., & Tranquillo, R. T. (2006). Cell sourcing and culture conditions for fibrin-based valve constructs. Tissue Engineering, 12(6), 1489–1502.
Jockenhoevel, S., Zund, G., Hoerstrup, S. P., Chalabi, K., Sachweh, J. S., Demircan, L., et al. (2001). Fibrin gel—Advantages of a new scaffold in cardiovascular tissue engineering. European Journal of Cardio-Thoracic Surgery, 19(4), 424–430.
Grassl, E. D., Oegema, T. R., & Tranquillo, R. T. (2003). A fibrin-based arterial media equivalent. Journal of Biomedical Materials Research. Part A, 66(3), 550–561.
Birla, R. K., Borschel, G. H., Dennis, R. G., & Brown, D. L. (2005). Myocardial engineering in vivo: Formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Engineering, 11(5–6), 803–813.
Linnes, M. P., Ratner, B. D., & Giachelli, C. M. (2007). A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials, 28(35), 5298–5306.
Wang, B., Borazjani, A., Tahai, M., Curry, A. L. D. J., Simionescu, D. T., Guan, J., et al. (2010). Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. Journal of Biomedical Materials Research. Part A, 94(4), 1100–1110.
Singelyn, J. M., DeQuach, J. A., Seif-Naraghi, S. B., Littlefield, R. B., Schup-Magoffin, P. J., & Christman, K. L. (2009). Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials, 30(29), 5409–5416.
Crapo, P. M., & Wang, Y. (2010). Small intestinal submucosa gel as a potential scaffolding material for cardiac tissue engineering. Acta Biomaterialia, 6(6), 2091–2096.
Robinson, K. A., Li, J., Mathison, M., Redkar, A., Cui, J., Chronos, N. A. F., et al. (2005). Extracellular matrix scaffold for cardiac repair. Circulation, 112(9 Suppl), I135–I143.
Gilbert, T. W., Sellaro, T. L., & Badylak, S. F. (2006). Decellularization of tissues and organs. Biomaterials, 27(19), 3675–3683.
Simpson, D. G., Terracio, L., Terracio, M., Price, R. L., Turner, D. C., & Borg, T. K. (1994). Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. Journal of Cellular Physiology, 161(1), 89–105.
Allison, D. D., & Grande-Allen, K. J. (2006). Review. Hyaluronan: A powerful tissue engineering tool. Tissue Engineering, 12(8), 2131–2140.
Ventura, C., Cantoni, S., Bianchi, F., Lionetti, V., Cavallini, C., Scarlata, I., et al. (2007). Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. Journal of Biological Chemistry, 282(19), 14243–14252.
Ruvinov, E., Leor, J., & Cohen, S. (2011). The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials, 32(2), 565–578.
Leor, J., Aboulafia-Etzion, S., Dar, A., Shapiro, L., Barbash, I. M., Battler, A., et al. (2000). Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation, 102(suppl III), III-56–III-61.
Xiang, Z., Liao, R., Kelly, M. S., & Spector, M. (2006). Collagen–GAG scaffolds grafted onto myocardial infarcts in a rat model: A delivery vehicle for mesenchymal stem cells. Tissue Engineering, 12(9), 2467–2478.
Li, R.-K., Jia, Z.-Q., Weisel, R. D., Mickle, D. A. G., Choi, A., & Yau, T. M. (1999). Survival and function of bioengineered cardiac grafts. Circulation, 100(Suppl II), II-63–II-69.
Malafaya, P. B., Silva, G. A., & Reis, R. L. (2007). Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews, 59(4–5), 207–233.
Gunatillake, P. A., & Adhikari, R. (2003). Biodegradable synthetic polymers for tissue engineering. European Cells & Materials, 5, 1–16.
Davis, M. E., Hsieh, P. C. H., Grodzinsky, A. J., & Lee, R. T. (2005). Custom design of the cardiac microenvironment with biomaterials. Circulation Research, 97(1), 8–15.
Kenar, H., Kose, G. T., & Hasirci, V. (2010). Design of a 3D aligned myocardial tissue construct from biodegradable polyesters. Journal of Materials Science. Materials in Medicine, 21(3), 989–997.
Park, H., Radisic, M., Lim, J. O., Chang, B. H., & Vunjak-Novakovic, G. (2005). A novel composite scaffold for cardiac tissue engineering. In Vitro Cellular & Developmental Biology—Animal, 41(7), 188–196.
Carrier, R. L., Papadaki, M., Rupnick, M., Schoen, F. J., Bursac, N., Langer, R., et al. (1999). Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnology and Bioengineering, 64(5), 580–589.
Caspi, O., Lesman, A., Basevitch, Y., Gepstein, A., Arbel, G., Habib, I. H. M., et al. (2007). Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circulation Research, 100(2), 263–272.
Engelmayr, G. C., Cheng, M., Bettinger, C. J., Borenstein, J. T., Langer, R., & Freed, L. E. (2008). Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Materials, 7(12), 1003–1010.
Wang, Y., Kim, Y. M., & Langer, R. (2003). In vivo degradation characteristics of poly(glycerol sebacate). Journal of Biomedical Materials Research, 66, 192–197.
Radisic, M., Park, H., Chen, F., Salazar-Lazzaro, J. E., Wang, Y., Dennis, R., et al. (2006). Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Engineering, 12(8), 2077–2091.
Shimizu, T. (2002). Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circulation Research, 90(3), 40e–48e.
Shin, M., Ishii, O., Sueda, T., & Vacanti, J. P. (2004). Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials, 25(17), 3717–3723.
Pego, A. P., Siebum, B., Van Luyn, M. J. A., Seijen, X. J. G. Y. V., Poot, A. A., Grijpma, D. W., et al. (2003). Preparation of degradable porous structures based on 1,3-trimethylene carbonate and d, l-lactide (co)polymers for heart tissue engineering. Tissue Engineering, 9(5), 981–994.
Blumenthal, B., Golsong, P., Poppe, A., Heilmann, C., Schlensak, C., Beyersdorf, F., et al. (2010). Polyurethane scaffolds seeded with genetically engineered skeletal myoblasts: A promising tool to regenerate myocardial function. Artificial Organs, 34(2), E46–E54.
Meng, J., Kong, H., Han, Z., Wang, C., Zhu, G., Xie, S., et al. (2009). Enhancement of nanofibrous scaffold of multiwalled carbon nanotubes/polyurethane composite to the fibroblasts growth and biosynthesis. Journal of Biomedical Materials Research. Part A, 88(1), 105–116.
Liao, I.-C., Liu, J. B., Bursac, N., & Leong, K. W. (2008). Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Experimental Cell Research, 1, 133–145.
Thomson, R., Wake, M., Yaszemski, M., & Mikos, A. (1995). Biodegradable polymer scaffolds to regenerate organs. Advances in Polymer Science, 122, 245–274.
Yang, S., Leong, K. F., Du, Z., & Chua, C. K. (2001). The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering, 7(6), 679–689.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260, 920–926.
Glowacki, J., & Mizuno, S. (2008). Collagen scaffolds for tissue engineering. Biopolymers, 89(5), 338–344.
Shapiro, L., & Cohen, S. (1997). Novel alginate sponges for cell culture and transplantation. Biomaterials, 18(8), 583–590.
Mikos, A. G., & Temenoff, J. S. (2000). Formation of highly porous biodegradable scaffolds for tissue engineering. Electronic Journal of Biotechnology, 3, 114–119.
Ott, H. C., Matthiesen, T. S., Goh, S.-K., Black, L. D., Kren, S. M., Netoff, T. I., et al. (2008). Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nature Medicine, 14(2), 213–221.
Badylak, S. F., Freytes, D. O., & Gilbert, T. W. (2009). Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia, 5(1), 1–13.
Rosso, F., Giordano, A., Barbarisi, M., & Barbarisi, A. (2004). From cell–ECM interactions to tissue engineering. Journal of Cellular Physiology, 199(2), 174–180.
Badylak, S. F., Taylor, D., & Uygun, K. (2011). Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annual Review of Biomedical Engineering, 13, (in press).
Zhong, S., Teo, W. E., Zhu, X., Beuerman, R. W., Ramakrishna, S., Yue, L., et al. (2006). An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. Journal of Biomedical Materials Research. Part A, 79(3), 456–463.
Blakeney, B. A., Tambralli, A., Anderson, J. M., Andukuri, A., Lim, D.-J., Dean, D. R., et al. (2011). Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials, 32(6), 1583–1590.
Sell, S. A., McClure, M. J., Garg, K., Wolfe, P. S., & Bowlin, G. L. (2009). Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Advanced Drug Delivery Reviews, 61(12), 1007–1019.
Szentivanyi, A., Chakradeo, T., Zernetsch, H., & Glasmacher, B. (2011). Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Advanced Drug Delivery Reviews, 63(4–5), 209–220.
Zong, X., Bien, H., Chung, C.-Y., Yin, L., Fang, D., Hsiao, B. S., et al. (2005). Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials, 26(26), 5330–5338.
Liu, H., Li, X., Zhou, G., Fan, H., & Fan, Y. (2011). Electrospun sulfated silk fibroin nanofibrous scaffolds for vascular tissue engineering. Biomaterials, 32(15), 3784–3793.
McManus, M. C., Boland, E. D., Simpson, D. G., Barnes, C. P., & Bowlin, G. L. (2006). Electrospun fibrinogen: Feasibility as a tissue engineering scaffold in a rat cell culture model. Journal of Biomedical Materials Research. Part A, 81(2), 299–309.
Ma, Z., Kotaki, M., Inai, R., & Ramakrishna, S. (2005). Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Engineering, 11(1–2), 101–109.
Drury, J. (2003). Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials, 24(24), 4337–4351.
Nicodemus, G. D., & Bryant, S. J. (2008). Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering. Part B, Reviews, 14(2), 149–165.
Bian, W., Liau, B., Badie, N., & Bursac, N. (2010). Mesoscopic hydrogel molding to control the 3D geometry of bioartifical muscle tissues. Nature Protocols, 4(10), 1522–1534.
Birla, R. K., Huang, Y. C., & Dennis, R. G. (2007). Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Engineering, 13(9), 2239–2248.
Lu, W.-N., Lü, S.-H., Wang, H.-B., Li, D.-X., Duan, C.-M., Liu, Z.-Q., et al. (2009). Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Engineering. Part A, 15(6), 1437–1447.
Krebs, M. D., Sutter, K. A., Lin, A. S. P., Guldberg, R. E., & Alsberg, E. (2009). Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering. Acta Biomaterialia, 5(8), 2847–2859.
Patterson, J., & Hubbell, J. A. (2010). Enhanced proteolytic degradation of molecularly engineered peg hydrogels in response to MMP-1 and MMP-2. Biomaterials, 31(30), 7836–7845.
Zhang, G., Wang, X., Wang, Z., Zhang, J., & Suggs, L. (2006). A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Engineering, 12(1), 9–19.
Zhang, G., Hu, Q., Braunlin, E. A., Suggs, L. J., & Zhang, J. (2008). Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Engineering. Part A, 14(6), 1025–1036.
Ruel-Gariépy, E., & Leroux, J.-C. (2004). In situ-forming hydrogels—Review of temperature-sensitive systems. European Journal of Pharmaceutics and Biopharmaceutics, 58(2), 409–426.
Ryan, E. (1999). Structural origins of fibrin clot rheology. Biophysical Journal, 77(5), 2813–2826.
Jia, X., & Kiick, K. L. (2009). Hybrid multicomponent hydrogels for tissue engineering. Macromolecular Bioscience, 9(2), 140–156.
Lee, K. Y., & Mooney, D. J. (2001). Hydrogels for tissue engineering. Chemical Reviews, 101(7), 1869–1880.
Akhyari, P., Kamiya, H., Haverich, A., Karck, M., & Lichtenberg, A. (2008). Myocardial tissue engineering: The extracellular matrix. European Journal of Cardio-Thoracic Surgery, 34(2), 229–241.
Anderson, J. M., Rodriguez, A., & Chang, D. T. (2008). Foreign body reaction to biomaterials. Seminars in Immunology, 20(2), 86–100.
Kushida, A., Yamato, M., Konno, C., Kikuchi, A., Sakurai, Y., & Okano, T. (1999). Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. Journal of Biomedical Materials Research, 45(4), 355–362.
Masuda, S., Shimizu, T., Yamato, M., & Okano, T. (2008). Cell sheet engineering for heart tissue repair. Advanced Drug Delivery Reviews, 60(2), 277–285.
Isenberg, B. C., Tsuda, Y., Williams, C., Shimizu, T., Yamato, M., Okano, T., et al. (2008). A thermoresponsive, microtexture substrate for cell sheet engineering with defined structural organization. Biomed Eng (NY), 29(17), 2565–2572.
Dike, L. E., Chen, C. S., Mrksich, M., Tien, J., Whitesides, G. M., & Ingber, D. E. (1999). Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cellular & Developmental Biology—Animal, 35(8), 441–448.
Guo, D., Kassiri, Z., & Oudit, G. Y. (2011). Role of signaling pathways in the myocardial response to biomechanical stress and in mechanotransduction in the heart. In A. Kamkin & I. Kiseleva (Eds.), Mechanosensitivity and mechanotransduction (pp. 141–166). Dordrecht: Springer.
Oyamada, M., Kimura, H., Yoamada, Y., Miyamoto, A., Ohshika, H., & Mori, M. (1994). The expression, phosphorylation, and localization of connexin 43 and gap-junctional intercellular communication during the establishment of a synchronized contraction of cultured neonatal rat cardiac myocytes. Experimental Cell Research, 212, 351–358.
Nichol, J. W., Engelmayr, G. C., Cheng, M., & Freed, L. E. (2008). Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochemical and Biophysical Research Communications, 373(3), 360–365.
Levenberg, S., Rouwkema, J., Macdonald, M., Garfein, E. S., Kohane, D. S., Darland, D. C., et al. (2005). Engineering vascularized skeletal muscle tissue. Nature Biotechnology, 23(7), 879–884.
Morritt, A. N., Bortolotto, S. K., Dilley, R. J., Han, X., Kompa, A. R., McCombe, D., et al. (2007). Cardiac tissue engineering in an in vivo vascularized chamber. Circulation, 115(3), 353–360.
Sasagawa, T., Shimizu, T., Sekiya, S., Haraguchi, Y., Yamato, M., Sawa, Y., et al. (2010). Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials, 31(7), 1646–1654.
Chen, Q., Harding, S., Ali, N., Lyon, A., & Boccaccini, A. (2008). Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater Sci Eng R Rep, 59(1–6), 1–37.
Papadaki, M., Bursac, N., Langer, R., Merok, J., Vunjak-Novakovic, G., & Freed, L. E. (2001). Tissue engineering of functional cardiac muscle: Molecular, structural, and electrophysiological studies. American Journal of Physiology. Heart and Circulatory Physiology, 280(1), H168–H178.
Bilodeau, K., & Mantovani, D. (2006). Bioreactors for tissue engineering: Focus on mechanical constraints. A comparative review. Tissue Engineering, 12(8), 2367–2383.
Radisic, M., Yang, L., Boublik, J., Cohen, R. J., Langer, R., Freed, L. E., et al. (2004). Medium perfusion enables engineering of compact and contractile cardiac tissue. American Journal of Physiology. Heart and Circulatory Physiology, 286(2), H507–H516.
Fink, C., Ergün, S., Kralisch, D., Remmers, U., Weil, J., & Eschenhagen, T. (2000). Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. The FASEB Journal, 14(5), 669–679.
Kraehenbuehl, T. P., Ferreira, L. S., Hayward, A. M., Nahrendorf, M., van der Vlies, A. J., Vasile, E., et al. (2010). Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials, 32(4), 1102–1109.
Perets, A., Baruch, Y., Weisbuch, F., Shoshany, G., Neufeld, G., & Cohen, S. (2003). Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. Journal of Biomedical Materials Research. Part A, 65(4), 489–497.
Ferreira, L. S., Gerecht, S., Fuller, J., Shieh, H. F., Vunjak-Novakovic, G., & Langer, R. (2007). Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials, 28(17), 2706–2717.
Sy, J. C., & Davis, M. E. (2010). Delivering regenerative cues to the heart: Cardiac drug delivery by microspheres and peptide nanofibers. Journal of Cardiovascular Translational Research, 3(5), 461–468.
Sapir, Y., Kryukov, O., & Cohen, S. (2011). Integration of multiple cell–matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials, 32(7), 1838–1847.
Spadaccio, C., Chello, M., Trombetta, M., Rainer, A., Toyoda, Y., & Genovese, J. A. (2009). Drug releasing systems in cardiovascular tissue engineering. Journal of Cellular and Molecular Medicine, 13(3), 422–439.
Leor, J., Amsalem, Y., & Cohen, S. (2005). Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacology and Therapeutics, 105(2), 151–163.
Chen, R. R., & Mooney, D. J. (2003). Polymeric growth factor delivery strategies for tissue engineering. Pharmaceutical Research, 20(8), 1103–1112.
Epstein, S. E., Kornowski, R., Fuchs, S., & Dvorak, H. F. (2001). Angiogenesis therapy: Amidst the hype, the neglected potential for serious side effects. Circulation, 104, 115–119.
Christoforou, N., & Gearhart, J. D. (2007). Stem cells and their potential in cell-based cardiac therapies. Progress in Cardiovascular Diseases, 49(6), 396–413.
Zhang, S. J., Truskey, G. A., & Kraus, W. E. (2007). Effect of cyclic stretch on beta1D-integrin expression and activation of FAK and RhoA. American Journal of Physiology. Cell Physiology, 292(6), C2057–C2069.
Rubbens, M. P., Driessen-Mol, A., Boerboom, R. A., Koppert, M. M. J., van Assen, H. C., TerHaar Romeny, B. M., et al. (2009). Quantification of the temporal evolution of collagen orientation in mechanically conditioned engineered cardiovascular tissues. Annals of Biomedical Engineering, 37(7), 1263–1272.
Akhyari, P., Fedak, P. W. M., Weisel, R. D., Lee, T.-Y. J., Mickle, D. A. G., & Li, R.-K. (2002). Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation, 106(suppl I), I-137–I-142.
Wille, J. J., Elson, E. L., & Okamoto, R. J. (2006). Cellular and matrix mechanics of bioartificial tissues during continuous cyclic stretch. Annals of Biomedical Engineering, 34(11), 1678–1690.
Asnes, C. F., Marquez, J. P., Elson, E. L., & Wakatsuki, T. (2006). Reconstitution of the Frank–Starling mechanism in engineered heart tissues. Biophysical Journal, 91(5), 1800–1810.
Tandon, N., Cannizzaro, C., Chao, P.-H. G., Maidhof, R., Ting, H., Au, H., et al. (2009). Electrical stimulation systems for cardiac tissue engineering. Nature Protocols, 4(2), 155–173.
Tandon, N., Marsano, A., Maidhof, R., Wan, L., Park, H., & Vunjak-Novakovic, G. (2011). Optimization of electrical stimulation parameters for cardiac tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 5(6), e115–e125.
Kavahagh, K. M., Duff, H. J., Clark, R., Robinson, K. V., Giles, W. R., & Wyse, D. G. (1990). Monophasic versus biphasic cardiac stimulation: Mechanism of decreased energy requirements. Pacing and Clinical Electrophysiology, 13(10), 1268–1276.
Huang, G., Pashmforoush, M., Chung, B., & Saxon, L. A. (2010). The role of cardiac electrophysiology in myocardial regenerative stem cell therapy. Journal of Cardiovascular and Translational Research, 4, 61–65.
Wikswo, J. P., Lin, S. F., & Abbas, R. A. (1995). Virtual electrodes in cardiac tissue: A common mechanism for anodal and cathodal stimulation. Biophysical Journal, 69(6), 2195–2210.
McDonough, P. M., & Glembotski, C. C. (1992). Induction of atrial natriuretic factor and myosin light chain-2 gene expression in cultured ventricular myocytes by electrical stimulation of contraction. Biochemistry, 267(17), 11665–11668.
Zimmermann, W.-H., Didié, M., Döker, S., Melnychenko, I., Naito, H., Rogge, C., et al. (2006). Heart muscle engineering: An update on cardiac muscle replacement therapy. Cardiovascular Research, 71(3), 419–429.
Iwakura, A., Fujita, M., Kataoka, K., Tambara, K., Sakakibara, Y., Komeda, M., et al. (2003). Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart and Vessels, 18(2), 93–99.
Davis, M. E., Hsieh, P. C. H., Takahashi, T., Song, Q., Zhang, S., Kamm, R. D., et al. (2006). Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 103(21), 8155–8160.
Seif-Naraghi, S. B., Salvatore, M. A., Schup-Magoffin, P. J., Hu, D. P., & Christman, K. L. (2010). Design and characterization of an injectable pericardial matrix gel: A potentially autologous scaffold for cardiac tissue engineering. Tissue Engineering. Part A, 16(6), 2017–2027.
Gaudette, G. R., & Cohen, I. S. (2006). Cardiac regeneration: Materials can improve the passive properties of myocardium, but cell therapy must do more. Circulation, 114(24), 2575–2577.
Mian, R., Morrison, W. A., Hurley, J. V., Penington, A. J., Romeo, R., Tanaka, Y., et al. (2000). Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Engineering, 6(6), 595–603.
Dvir, T., Kedem, A., Ruvinov, E., Levy, O., Freeman, I., Landa, N., et al. (2009). Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proceedings of the National Academy of Sciences of the United States of America, 106(35), 14990–14995.
Kelm, J. M., & Fussenegger, M. (2010). Scaffold-free cell delivery for use in regenerative medicine. Advanced Drug Delivery Reviews, 62(7–8), 753–764.
Shimizu, T., Sekine, H., Yang, J., Isoi, Y., Yamato, M., Kikuchi, A., et al. (2006). Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. The FASEB Journal, 20(6), 708–710.
Giraud, M.-N., Ayuni, E., Cook, S., Siepe, M., Carrel, T. P., & Tevaearai, H. T. (2008). Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artificial Organs, 32(9), 692–700.
Dimmeler, S., & Zeiher, A. M. (2009). Cell therapy of acute myocardial infarction: Open questions. Cardiology, 113(3), 155–160.
Haider, H. K., & Ashraf, M. (2010). Preconditioning and stem cell survival. Journal of Cardiovascular Translational Research, 3(2), 89–102.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, K.Y., Black, L.D. Strategies for Tissue Engineering Cardiac Constructs to Affect Functional Repair Following Myocardial Infarction. J. of Cardiovasc. Trans. Res. 4, 575–591 (2011). https://doi.org/10.1007/s12265-011-9303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9303-1