Abstract
Delayed cerebral ischemia after subarachnoid hemorrhage (SAH) may be affected by a number of factors, including cerebral blood flow and oxygen delivery. Anemia affects about half of patients with SAH and is associated with worse outcome. Anemia also may contribute to the development of or exacerbate delayed cerebral ischemia. This review was designed to examine the prevalence and impact of anemia in patients with SAH and to evaluate the effects of transfusion. A literature search was made to identify original research on anemia and transfusion in SAH patients. A total of 27 articles were identified that addressed the effects of red blood cell transfusion (RBCT) on brain physiology, anemia in SAH, and clinical management with RBCT or erythropoietin. Most studies provided retrospectively analyzed data of very low-quality according to the GRADE criteria. While RBCT can have beneficial effects on brain physiology, RBCT may be associated with medical complications, infection, vasospasm, and poor outcome after SAH. The effects may vary with disease severity or the presence of vasospasm, but it remains unclear whether RBCTs are a marker of disease severity or a cause of worse outcome. Erythropoietin data are limited. The literature review further suggests that the results of the Transfusion Requirements in Critical Care Trial and subsequent observational studies on RBCT in general critical care do not apply to SAH patients and that randomized trials to address the role of RBCT in SAH are required.
Similar content being viewed by others
Introduction
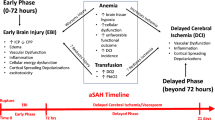
Aneurysm rupture causing subarachnoid hemorrhage (SAH) occurs in about 10/100,000 people each year [1, 2]. Nearly half of these individuals are dead within 30 days [1–5]. Among survivors, only one-third make a full recovery and approximately half who appear to experience a favorable outcome have neuropsychological and cognitive deficits and difficulties in their daily activities [6–10]. Poor outcome after SAH may be associated with two preventable factors delayed cerebral ischemia (DCI) and extracerebral organ dysfunction (e.g., medical complications and infection) [11–19]. The mortality rate from extracerebral organ dysfunction is 20–40% [17, 18]. DCI occurs in about 30% of patients, is often associated with arterial vasospasm that begins about 3 days after SAH, is maximal days 7–8, resolves after 14 days, and is identified radiographically in about 70% of patients [20–24]. Clinical trials to prevent vasospasm seldom have improved clinical outcome, despite reduced vessel narrowing [21, 25, 26]. This dissociation between clinical outcome and vasospasm has refocused efforts to limit brain injury rather than vessel narrowing and has renewed interest in intensive care strategies to prevent DCI and medical complications.
DCI is caused by impaired cerebral blood flow (CBF) and O2 delivery (DO2). Cerebral circulation compensates for reduced CBF by increasing oxygen extraction fraction (OEF) to maintain the amount of oxygen available for metabolism and to prevent ischemia. When OEF is increased (oligemia), tissues may not compensate for further DO2 reductions and, if not corrected, infarction may occur. Therefore, avoiding critical DO2 reductions is central to SAH care. CBF and CaO2 determine cerebral DO2, and since hemoglobin (Hb) levels primarily determine CaO2, anemia may impair cerebral DO2. After SAH, more than half the patients develop anemia [27, 28] that maybe associated with worse outcome. Clinical studies also suggest that Hb > 11 g/dL may be associated with improved SAH outcome [29–31]. However, higher Hb may increase blood viscosity and, with autoregulatory vasoconstriction in response to increased CaO2, further reduce CBF, countering any DO2 benefit. Furthermore, RBCT (red blood cell transfusion) has been associated with organ dysfunction and mortality [32–35]. This effect may be mediated by inflammatory mediators or altered nitric oxide (NO) metabolism among other factors in transfused cells [36–41]. Both inflammation and NO influence vasospasm [21, 42]. Limited data are available to guide anemia management and RBCT after SAH.
This literature review was designed to present available published evidence on the occurrence and outcome of anemia in SAH patients. The role of RBCT and erythropoietin on brain physiology and clinical outcome also was explored.
Methods
A literature search was made to identify clinical or experimental studies published between 1980 and August 2010 in the English literature that described and compared RBCT strategies and Hb levels after rupture of a cerebral aneurysm. Candidate articles were identified from electronic databases, including Medline and EMBASE, Index Medicus, bibliographies of pertinent articles, and expert consultation. Additional articles were identified through review of textbooks, bibliographies from retrieved articles, and the “Related Articles” feature of PubMed. For electronic searches, the following key words were used: “subarachnoid hemorrhage,” “subarachnoid hemorrhage outcome,” “anemia,” “hemoglobin,” “transfusion,” “packed red blood cells,” “vasospasm,” “delayed cerebral ischemia,” “blood products,” and “erythropoietin.” This search was supplemented by also identifying randomized trials that have compared transfusion strategies in general critical care, recent review articles on transfusion in neurocritical care, transfusion guidelines, and studies that have evaluated transfusion and hemoglobin in traumatic brain injury (TBI).
Original research studies were selected for detailed review if they addressed incidence and/or outcome of anemia and treatment with RBCT or erythropoietin after SAH. Selected studies were evaluated for quality of evidence using the GRADE system [43].
Summary of the Literature
Five hundred and twelve manuscripts were identified. There is no high-quality evidence to support a particular transfusion strategy or Hb level in patients with SAH. Twenty-seven articles were selected for detailed review (Tables 1, 2, 3, 4) [27, 29–31, 44–66]. These studies addressed brain physiology related to transfusion or Hb (11 articles—5 in traumatic brain injury and 6 in SAH; Table 1), anemia in SAH (5 articles; Table 2), RBCT management in SAH (8 articles; Table 3), and erythropoietin after aneurysm rupture (4 articles; Table 4). Most studies describe retrospective analyses of clinical series. Overall, the quality of evidence is very low according to the GRADE criteria. One small, randomized pilot study evaluated two transfusion strategies in SAH [58]; however, this study was underpowered, and no conclusions about a particular strategy could be made. The study, however, suggests that a randomized trial is safe and feasible.
The data summarized in the tables support that anemia affects about half of patients with SAH and is linked with worsened outcome. While RBCT has been shown to have beneficial effects on brain physiology, RBCT is associated with medical complications, infection, vasospasm, and poor outcome after SAH. It remains unclear whether RBCTs are simply a marker of disease severity or an independent cause of worse outcome. Erythropoietin data are very limited.
In addition to those articles meeting criteria for detailed review, additional publications provide clinically useful information on the potential role of RBCT in SAH. These studies are summarized below.
Anemia After SAH
Anemia is common after SAH. Depending on the definition applied, anemia has been identified in 40–50% of SAH patients and only 16% maintain Hb > 11 g/dL [27, 54, 55, 67]. The mean drop in Hb after SAH is 3 g/dL, and anemia develops after a mean of 3.5 days [27]. Anemia may exacerbate the reduction in oxygen delivery that underlies DCI. Observational studies have linked anemia or a larger Hb reduction with infarction, dependency, and death after SAH [19, 29, 55, 68]. In addition, patients with an unfavorable outcome consistently have lower Hb levels, especially between days 6 and 11, following SAH (i.e., during the greatest risk period for DCI) [55].
Experimental evidence links anemia with reduced PbtO2 and increased neuron injury after acute brain injury [69–71]. In the normal brain, compensatory vasodilation occurs with Hb < 10 g/dL [72], so brain hypoxia usually is manifest only at lower Hb levels (e.g., <6 g/dL) [73]. When cerebrovascular reserve is impaired, e.g., in patients with SAH, tissue hypoxia and cell injury may develop at a higher Hb. For example, using cerebral microdialysis in 20 poor-grade SAH patients, Hb ≤ 9 g/dL was identified as an independent factor associated with cerebral tissue injury [53]. In a similar study in which patients requiring FiO2 > 60% were excluded, Kurtz et al. linked Hb < 10 g/dL with cell energy dysfunction [54]. Consistent with this, mathematical modeling based on animal experiments of brain ischemia suggests that Hb < 10 g/dL is associated with brain hypoxia [71].
Correction of anemia with RBCT may, therefore, improve PbtO2 and attenuate cell damage. In SAH patients, anemia can be associated with poor outcome, and avoidance of low Hb may, therefore, be warranted [56]. The optimal Hb threshold for RBCT in SAH patients remains unclear although a recent clinical study suggests that Hb > 11 g/dL is associated with less cerebral infarction and improved outcome after SAH [30].
RBCT in General Medical and Surgical Critical Care
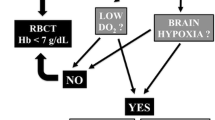
In most cases, RBCT is used in critical care for the treatment of anemia [74, 75], with a commonly used Hb cutoff of 10 g/dL to augment oxygen delivery and avoid oxygen debt [76]. This practice is now challenged by evidence that suggests RBCT may exacerbate outcome and increase medical complications in general critical care [32–34]. Consequently, a restrictive RBCT policy (Hb ~ 7 g/dl) may be preferred.
In general critical care patients, RBCT is associated with complications such as immunosuppression, transmission of infectious agents, postoperative infections, and pneumonia [77–83]. RBCT also is an independent risk factor for impaired pulmonary function and prolonged ventilator support, acute lung injury, acute respiratory distress syndrome (ARDS) [84, 85], systemic inflammatory response syndrome [83, 86], renal dysfunction [87], multiple organ failure or dysfunction [34, 88, 89], transfusion reactions [39], and increased length of stay [33]. In SAH patients, RBCT has also been associated with medical complications and infection [29, 57].
Recent observational data suggest that many intensive care patients can tolerate Hb of 7 g/dL, “restrictive” RBCT is safe, or that RBCT may exacerbate outcome or increase complications [35, 74, 80, 89]. These studies, however, included few if any patients with neurological disorders or SAH. There is a dose effect, but as little as one unit of blood may be deleterious [90]. Two randomized trials, one in adults [32] and one in children [91], have addressed RBCT in critical care. The Transfusion Requirements in Critical Care Trial (TRICC) compared a “liberal (10 g/dL)” and “restricted (7 g/dL)” RBCT trigger in 838 ICU patients [32]. Overall 30-day mortality was similar, with lower mortality in the restrictive RBCT group among younger (<55 years) and less ill (APACHE II < 20) patients. Concern remains, however, that restrictive RBCT may not be tolerated in patients with some conditions, e.g., reduced cerebrovascular or cardiac reserve. Consequently, patients with ARDS, sepsis, myocardial ischemia, or traumatic brain injury may require higher Hb levels [92–94]. For example, there may be a benefit to liberal RBCT in elderly patients, with acute coronary syndrome and admission Hb < 8 g/dL [93, 94]. The CRUSADE initiative data from 44,242 patients with non-ST segment elevation acute coronary syndrome suggest that the association between RBCT and outcomes was a function of nadir hematocrit [95]. RBCT tended to have a beneficial impact with hematocrit <25%, with increased mortality when nadir hematocrit was >27%. Conversely, liberal RBCT does not appear to benefit patients who require prolonged mechanical ventilation, where theoretically the oxygen carrying benefit of RBCT might hasten recovery [96, 97]. Together, these various data suggest the effect of RBCT on outcome may depend on the need for oxygen delivery and on the individual patient and his or her pathology.
RBCT in SAH
Retrospective studies report that about one-quarter of patients with SAH receive RBCT during surgery and up to two-thirds during their intensive care stay [31, 98–100]. The first RBCT during intensive care is generally administered a mean of 4.6 days after SAH [31], i.e., just as vasospasm becomes maximal.
There are good theoretical reasons to maintain a higher Hb after brain injury, since the brain has stringent O2 requirements. Most neurosurgeons prefer Hb > 10 g/dL for patients with acute brain injury to maintain optimal oxygen carrying capacity; however, there is variability in the response of brain tissue O2 (PbtO2) to RBCT [44]. Even when RBCT improves PbtO2, it is unclear whether this improves brain metabolism [47]. Few studies have investigated RBCT effects on outcome after brain injury. Subset analysis of 67 traumatic brain injury patients enrolled in the TRICC trial [101] suggests TBI patients can have a similar RBCT threshold to other intensive care patients, while observational data suggest RBCT is associated with worse outcome in this population [102–104]. Many SAH patients, unlike traumatic injury patients, have associated cardiac dysfunction [105], a relative contraindication to restrictive RBCT suggesting that RBCT in SAH needs specific study. PET studies, for example, show that RBCT can improve CaO2 and DO2 without a detrimental effect on CBF in SAH patients with anemia [51].
Data evaluating an effect of RBCT in patients with SAH are limited, despite fluid status and oxygenation being critical to patient care. Not all studies demonstrate an association between RBCT and poor outcome after SAH [59, 61]. However, recent observational studies and post hoc analysis of other trials link liberal use of RBCT with medical complications, infection, vasospasm, poor cognitive performance, and poor outcome [29, 44, 55–57, 60, 68]. It is conceivable that avoiding hypoxia, rather than anemia alone, may prevent neuron damage [106]. RBCT, however, does not always increase PbtO2, and in 20–25% of patients, PbtO2 may decrease [44, 46]. A plausible biologic explanation for the deleterious effects of RBCT is that stored red blood cells have been associated with immunomodulation, impaired vasoregulation, hypercoagulation, altered nitric oxide metabolism, reduced red blood cell deformability, altered red blood cell adhesiveness and aggregability, reduced 2,3-diphosphoglycerate, and impaired microvascular perfusion [36–39]. Some of these factors appear integral to vasospasm [21, 107, 108].
Clinical Management Strategies for Anemia in SAH
A recent international survey queried intensive care physicians about SAH care [109]. Among the 626 respondents, recommendations for optimal Hb ranged from ~8 g/dL (25%) to ~12–13 g/dL (40%). Two-thirds advocated a target Hb > 10 g/dL. There is also widespread variation in the use of RBCT in treating SAH patients, although practices differ from those used in general surgical and medical conditions [110]. Discrepancies identified in clinical practice highlight the need for more research to specifically identify the role of anemia and anemia management in patients with SAH. In addition, there remain many unanswered questions about transfusion after SAH including the role of the following: (1) plasma and platelet component therapies, (2) leukocyte reduction, (3) age of transfused cells, (4) blood product substitutes, and (5) the clinical or biological end point for RBCT. There has been limited study of erythropoietin use, and no firm recommendations about its use can be made.
Conclusions
Anemia develops in about 50% of SAH patients and often within 3 days of aneurysm rupture. Risk factors for anemia after SAH include female sex, advanced age, worse clinical grade, lower admission Hb, and surgery. Anemia has also been identified as a risk factor for poor outcome after SAH. It is not clear whether anemia is an independent factor associated with outcome or a marker of disease severity. However, patients in worse clinical grade or those who develop vasospasm are more likely to have a worse outcome if they develop anemia.
There is limited information about how often SAH patients require RBCT, but recent retrospective studies demonstrate that about one-quarter receive RBCT during surgery and up to two-thirds during their intensive care stay. Physiological studies show increases in brain oxygen in 75% of transfusions and increases of brain DO2. RBCT, however, have been associated with vasospasm, medical complications, infections, worse outcome, and cognitive impairment. When both anemia and RBCT are entered into outcome models, transfusion has a greater effect. Again, it is not clear whether RBCT is a marker of disease severity or an independent risk factor for worse outcome. While the overall quality of literature that examines transfusion in SAH is low, it is clear that the results of the TRICC trial and subsequent observational studies of transfusion in general critical care do not and should not apply to SAH patients. For now, clinicians will need to base transfusion decisions for SAH patients in the context of conflicting information and so should focus on an individualized assessment of anemia tolerance, consider blood conservation strategies, and understand the potential risks and benefits of blood transfusion. Further prospective investigations to address the role of anemia, the optimal Hb threshold, and the use of RBCT in SAH are desperately needed.
References
ACROSS Group. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke. 2000;31:1843–50.
Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054–61.
Broderick JP, Thomas GB, Dudler JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–7.
Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage: a population-based study. Stroke. 1993;24:1649–54.
Le Roux P, Winn HR. Management of the ruptured aneurysm. In: Le Roux P, Winn HR, Newell DW, editors. Management of cerebral aneurysms. Philadelphia: Elsevier; 2004. p. 303–33.
Kreiter KT, Copeland D, Bernardini GL, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33:200–8.
Ogden JA, Mee EW, Henning M. A prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery. 1993;33:572–87.
Ropper AH, Zervas NT. Outcome one year after SAH from cerebral aneurysm. Management morbidity, mortality and functional status in 112 consecutive good risk patients. J Neurosurg. 1984;60:909–15.
Tidswell P, Dias PS, Sagar HJ, Mayes AR, D Phil, Battersby RDE. Cognitive outcome after aneurysm rupture: relationship to aneurysm site and perioperative complications. Neurology 1995;45:875–82.
Scharbrodt W, Stein M, Schreiber V, Böker DK, Oertel MF. The prediction of long-term outcome after subarachnoid hemorrhage as measured by the Short Form-36 Health Survey. J Clin Neurosci. 2009;16:1409–13.
Claassen J, Vu A, Kreiter KT, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32:832–8.
Gruber A, Reinprecht A, Illievich UM, et al. Extracerebral organ dysfunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit Care Med. 1999;27:505–14.
Le Roux PD, Elliott JP, Downey L, et al. Improved outcome after rupture of anterior circulation aneurysms: a retrospective 10-year review of 224 good-grade patients. J Neurosurg. 1995;83:394–402.
Le Roux PD, Elliott JP, Newell D, et al. Predicting outcome in poor grade patients with subarachnoid hemorrhage: a review of 159 aggressively managed cases. J Neurosurg. 1996;85:39–49.
Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410–6.
Schuiling WJ, Dennesen PJ, Rinkel GJ. Extracerebral organ dysfunction in the acute stage after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;3:1–10.
Solenski N, Haley C, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Crit Care Med. 1995;23:1007–17.
Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23.
Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36.
Jahromi BS, MacDonald RL. Vasospasm: diagnosis and medical management. In: Le Roux P, Winn HR, Newell DW, editors. Management of cerebral aneurysms. Philadelphia: Elsevier; 2004. p. 455–87.
Dorsch NWC, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part 1: Incidence and effects. J Clin Neurosci. 1994;1:19–26.
Haley EC Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneuriymal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–74.
Lanzino G, Kassell NJ. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–24.
Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21.
Kreiter KT, Mayer SA, Howard G, Knappertz V, Ilodigwe D, Sloan MA, Macdonald RL. Sample size estimates for clinical trials of vasospasm in subarachnoid hemorrhage. Stroke. 2009;40:2362–7.
Sampson TR, Dhar R, Diringer MN. Factors associated with the development of anemia after subarachnoid hemorrhage. Neurocrit Care. 2010;12:4–9.
Giller CA, Wills MJ, Giller AM, Samson D. Distribution of hematocrit values after aneurysmal subarachnoid hemorrhage. J Neuroimag. 1998;8:169–70.
Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med. 2008;36:2070–5.
Naidech AM, Jovanovic B, Wartenberg KE, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35:2383–9.
Naidech AM, Drescher J, Ault ML, Shaibani A, Batjer HH, Alberts MJ. Higher hemoglobin is associated with less cerebral infarction, poor outcome, and death after subarachnoid hemorrhage. Neurosurgery. 2006;59:775–80.
Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409–17 (Erratum in: N Engl J Med 1999;340:1056).
Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905.
Moore FA, Moore EE, Sauaia A. Blood transfusion: an independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–5.
Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74.
Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–9.
Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–73.
Fransen E, Maessen J, Senden N, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–9.
Blajchman MA. Transfusion-associated immunomodulation and universal white cell reduction: are we putting the cart before the horse? Transfusion. 1999;39:665–70.
Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12.
Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–7.
Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–33.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Smith MJ, Maggee S, Stiefel M, Bloom S, Gracias V, Le Roux P. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–8.
Leal-Noval SR, Rincón-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med. 2006;32:1733–40.
Leal-Noval SR, Muñoz-Gómez M, Arellano-Orden V, et al. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–6.
Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37:1074–8.
Figaji AA, Zwane E, Kogels M, et al. The effect of blood transfusion on brain oxygenation in children with severe traumatic brain injury. Pediatr Crit Care Med. 2010;11:325–31.
Ekelund A, Reinstrup P, Ryding E, et al. Effects of iso- and hypervolemic hemodilution on regional cerebral blood flow and oxygen delivery for patients with vasospasm after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2002;144:703–12.
Muench E, Horn P, Bauhuf C, Roth H, Philipps M, Hermann P, Quintel M, Schmiedek P, Vajkoczy P. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med. 2007;35:1844–51.
Dhar R, Zazulia AR, Videen TO, Zipfel GJ, Derdeyn CP, Diringer MN. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke. 2009;40:3039–44.
Naidech AM, Bendok BR, Ault ML, Bleck TP. Monitoring with the Somanetics INVOS 5100C after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2008;9:326–31.
Oddo M, Milby A, Chen I, et al. Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage: a microdialysis study. Stroke. 2009;40:1275–81.
Kurtz P, Schmidt JM, Claassen J, et al. Anemia is associated with metabolic distress and brain tissue hypoxia after subarachnoid hemorrhage. Neurocrit Care. 2010;13:10–6.
Kramer AH, Zygun DA, Bleck TP, Dumont AS, Kassell NF, Nathan B. Relationship between hemoglobin concentrations and outcomes across subgroups of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;10:157–65.
Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage: new strategies for prevention and management. Curr Opin Crit Care. 2006;12:78–84.
Levine J, Kofke A, Cen L, Chen Z, Elliott JP, Winn HR, Le Roux P. Red blood cell transfusion is associated with medical complications after subarachnoid hemorrhage. Neurosurgery. 2010;66:312–8.
Naidech AM, Shaibani A, Garg RK, et al. Prospective, randomized trial of higher goal hemoglobin after subarachnoid hemorrhage. Neurocrit Care. 2010;13:313–20.
Broessner G, Lackner P, Hoefer C, et al. Influence of red blood cell transfusion on mortality and long-term functional outcome in 292 patients with spontaneous subarachnoid hemorrhage. Crit Care Med. 2009;37:1886–92.
Springer MV, Schmidt JM, Wartenberg KE, Frontera JA, Badjatia N, Mayer SA. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery. 2009;65:1043–50.
Tseng MY, Hutchinson PJ, Kirkpatrick PJ. Effects of fluid therapy following aneurysmal subarachnoid haemorrhage: a prospective clinical study. Br J Neurosurg. 2008;22:257–68.
Smith MJ, Le Roux PD, Elliott JP, Winn HR. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101:1–7.
Tseng MY, Hutchinson PJ, Kirkpatrick PJ. Interaction of neurovascular protection of erythropoietin with age, sepsis, and statin therapy following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:1235–9.
Tseng MY, Hutchinson PJ, Richards HK, et al. Acute systemic erythropoietin therapy to reduce delayed ischemic deficits following aneurysmal subarachnoid hemorrhage: a Phase II randomized, double-blind, placebo-controlled trial. J Neurosurg. 2009;111:171–80.
Springborg JB, Møller C, Gideon P, Jørgensen OS, Juhler M, Olsen NV. Erythropoietin in patients with aneurysmal subarachnoid haemorrhage: a double blind randomised clinical trial. Acta Neurochir (Wien). 2007;149:1089–101.
Springborg JB, Sonne B, Frederiksen HJ, et al. Erythropoietin in the cerebrospinal fluid of patients with aneurysmal subarachnoid haemorrhage originates from the brain. Brain Res. 2003;984:143–8.
Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89.
Tseng MY, Hutchinson PJ, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute pravastatin treatment on intensity of rescue therapy, inpatient hospital stay and six- month outcome in patients after aneurhemorrhage. Stroke. 2007;38:1545–50.
Hare GM, Mazer CD, Mak W, et al. Hemodilutional anemia is associated with increased cerebral neuronal nitric oxide synthase gene expression. J Appl Physiol. 2003;94:2058–67.
Johannson H, Siesjo BK. Brain energy metabolism in anesthetized rats in acute anemia. Acta Physiol Scand. 1975;93:515–24.
Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth. 1997;79:346–51.
Borgstrom L, Johannsson H, Siesjo BK. The influence of acute normovolemic anemia on cerebral blood flow and oxygen consumption of anesthetized rats. Acta Physiol Scand. 1975;93:505–14.
McLaren AT, Marsden PA, Mazer CD, et al. Increased expression of hif-1alpha, nnos, and vegf in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R403–14.
Corwin HL, Gettinger A, Pearl RG, et al. Anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32:39–52.
Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–57.
Russell JA, Phang PT. The oxygen delivery/consumption controversy: an approach to management of the critically ill. Am J Respir Crit Care Med. 1994;149:533–7.
Palmieri TL, Caruso DM, Foster KN, et al. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006;34:1602–7.
Taylor RW, Manganaro L, O’Brien J, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–54.
Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–90.
Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16.
Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–14.
Shorr AF, Duh MS, Kelly KM, Kollef MH, CRIT study group. Red blood cell transfusion and ventilator-associated pneumonia: a potential link? Crit Care Med. 2004;32:666–74.
Earley AS, Gracias VH, Haut E, et al. Anemia management program reduces transfusion volumes, incidence of ventilator-associated pneumonia, and cost in trauma patients. J Trauma. 2006;61:1–5.
Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8.
Zilberberg MD, Carter C, Lefebvre P, et al. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: a cohort study. Crit Care. 2007;11:R63.
Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45.
Ranucci M, Pavesi M, Mazza E, et al. Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion. 1994;9:319–26.
Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140:432–8.
Vincent JL, Baron J-F, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. J Am Med Assoc. 2002;288:1499–507.
Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31(12 Suppl):S687–97.
Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19.
Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH. Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med. 2009;35:480–8.
Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–6.
Aronson D, Dann EJ, Bonstein L, et al. Impact of RBC transfusion on clinical outcomes in patients with acute myocardial infarction. Am J Cardiol. 2008;102:115–9.
Alexander KP, Chen AY, Want TY, et al. Transfusion practice and outcomes in non- ST-segment elevation acute coronary syndromes. Am Heart J. 2008;155:1047–53.
Hebert PC, Blajchman MA, Cook DJ, et al. Do blood transfusions improve outcomes related to mechanical ventilation? Chest. 2001;119:1850–7.
Levy MM, Abraham E, Zilberberg M, et al. A descriptive evaluation of transfusion practices in patients receiving mechanical ventilation. Chest. 2005;127:928–35.
Couture DE, Ellegala DB, Dumont AS, Mintz PD, Kassell NF. Blood use in cerebrovascular neurosurgery. Stroke 2002;33:994–7. (Erratum in Stroke 2002;33:1442).
Le Roux P, Elliott JP, Winn HR. Blood transfusion during aneurysm surgery. Neurosurgery. 2001;49:1068–75.
de Gray LC, Matta BF. The health economics of blood use in cerebrovascular aneurysm surgery: the experience of a UK centre. Eur J Anaesthesiol. 2005;22:925–8.
McIntyre L, Hebert PC, Wells G, et al. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? J Trauma. 2004;57:563–8.
Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma. 2006;61(3):567–71.
Duane TM, Mayglothling J, Grandhi R, et al. The effect of anemia and blood transfusions on mortality in closed head injury patients. J Surg Res. 2008;147:163–7.
Salim A, Hadjizacharia P, DuBose J, et al. Role of anemia in traumatic brain injury. J Am Coll Surg. 2008;207:398–406.
Mayer SA, Lin J, Homma S, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–6.
Shimojyo S, Scheinberg P, Kogure K, Reinmuth OM. The effects of graded hypoxia upon transient cerebral blood flow and oxygen consumption. Neurology. 1968;18:127–33.
Stein S, Levine J, Nagpal S, Le Roux P. Vasospasm as the sole cause of cerebral ischemia: how strong is the evidence? Neurosurg Focus. 2006;21:E1–7.
Pluta RM, Zhang J, Dreier J, et al. Cerebral vasospasm after subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–8.
Stevens RD, Naval NS, Mirski MA, Citerio G, Andrews PJ. Intensive care of aneurysmal subarachnoid hemorrhage: an international survey. Intensive Care Med. 2009;35:1556–66.
Kramer AH, Diringer MN, Suarez JI, Naidech AM, Macdonald RL, Le Roux P. Red blood cell transfusion in aneurysmal subarachnoid hemorrhage patients: a multidisciplinary North American survey. Neurocritical Care Society Meeting, San Francisco, CA, 2010. Available at http://www.neurocriticalcare.org/i4a/pages/index.cfm?pageid=3719. Accessed April 2011.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage: Michael N. Diringer, Thomas P. Bleck, Nicolas Bruder, E. Sander Connolly, Jr., Giuseppe Citerio, Daryl Gress, Daniel Hanggi, J. Claude Hemphill, III, MAS, Brian Hoh, Giuseppe Lanzino, Peter Le Roux, David Menon, Alejandro Rabinstein, Erich Schmutzhard, Lori Shutter, Nino Stocchetti, Jose Suarez, Miriam Treggiari, MY Tseng, Mervyn Vergouwen, Paul Vespa, Stephan Wolf, Gregory J. Zipfel.
Rights and permissions
About this article
Cite this article
Le Roux, P.D., The Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Anemia and Transfusion After Subarachnoid Hemorrhage. Neurocrit Care 15, 342–353 (2011). https://doi.org/10.1007/s12028-011-9582-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9582-z