Abstract
Objective
To investigate the long-term influence of erythrocyte transfusion on cerebral oxygenation in patients with severe traumatic brain injury.
Design
Prospective and observational study.
Setting
Neurotrauma intensive care unit of trauma center level I.
Patients
Sixty consecutive, hemodynamically stable patients with severe traumatic brain injury, pretransfusion hemoglobin < 100 g/l, non-bleeding and monitored through intracranial pressure and brain tissue partial pressure of oxygen (PtiO2) catheters were included.
Interventions
Transfusion of 1–2 units of red blood cells.
Measurements and results
Ten sets of variables (pretransfusion, end of transfusion, and 1, 2, 3, 4, 5, 6, 12 and 24 h after transfusion) were recorded, including: PtiO2, cerebral perfusion pressure (CPP), end-tidal CO2, peripheral saturation of oxygen, temperature, hemoglobin, lactate and PaO2/FiO2 ratio. Transfusion was associated with an increase in PtiO2 during a 6-h period, with a peak at 3 h (26.2%; p = 0.0001) in 78.3% of the patients. No relationship was observed between PtiO2, CPP and hemoglobin increments. The relative increment in PtiO2 at hour 3 was only correlated with baseline PtiO2 (r2 0.166; p = 0.001). All of the patients with basal PtiO2 < 15 mmHg showed an increment in PtiO2 versus 74.5% of patients with basal PtiO2 ≥ 15 mmHg (p < 0.01, hour 3).
Conclusions
Erythrocyte transfusion is associated with a variable and prolonged increment of cerebral tissue oxygenation in anemic patients with severe traumatic brain injury. Low baseline PtiO2 levels (< 15 mmHg) could define those patients who benefit the most from erythrocyte transfusion.
Similar content being viewed by others
Introduction
Anemia is highly prevalent in the ICU [1], and a great proportion of critically ill patients are transfused. However, transfusion of allogeneic erythrocytes has independently been associated with both a longer stay at the ICU and overall hospital stay, as well as with higher rates of mortality and morbidity [2, 3, 4]. There is a considerable lack of data supporting the efficacy of transfusion in facilitating the uptake of oxygen into the tissues [5, 6, 7], thus increasing tissue oxygen consumption, the main admitted indication for blood transfusion.
Very little evidence exists regarding transfusion recommendations in neurological intensive care. Some observational studies carried out in patients with acute ischemic stroke and subarachnoid hemorrhage have shown a direct relationship between transfusion and poor clinical outcome [8, 9, 10]. Therefore, assessing the effect of blood transfusion on cerebral oxygenation could be crucial, before subjecting a patient to the risks of transfusion.
Brain tissue oxygen partial pressure (PtiO2) has successfully been used to recognize critical episodes of hypoxia or cerebral ischemia. This technique has been validated [11, 12] and, therefore, could be a good diagnostic tool for the demonstration of transfusion effects on oxygen uptake into the tissues. In a recent study of 35 patients with severe traumatic brain injury (TBI) and subarachnoid hemorrhage who had anemia, transfusion of packed red blood cells (RBC) increased PtiO2 in 75% of the patients [13]. However, the long-term effects of transfusion on PtiO2 were not assessed in this study. This is of crucial importance, since 2,3-DPG levels usually take several hours to reach adequate concentrations [14], so changes in tissue oxygenation following erythrocyte transfusion should be monitored for a prolonged time.
We hypothesized that transfusion of erythrocytes (RBCT) produces long-term increases in cerebral oxygenation in patients with severe TBI. The aim of our work was to investigate blood transfusion-related variations in PtiO2 during a 24-h period in stable patients with severe TBI. Additionally, we aimed to establish whether baseline values of PtiO2 and other variables might influence blood transfusion-related variations in PtiO2.
Material and methods
Setting and patients
This prospective and observational study was conducted between 1 July 2003 and 31 March 2005 at the Neurotrauma Intensive Care Unit (22 beds) of the public teaching hospital “Virgen del Rocio”, Seville, Spain, which is a trauma center level I with capacity for 2,000 beds. The institutional review board approved this study and waived the need for informed consent.
Patients with severe TBI (Glasgow Coma Scale score ≤ 8), fulfilling all of the following criteria, were included: (1) having an intraparenchymal ICP/PtiO2 catheter previously inserted; (2) having passed the initial resuscitation phase; (3) no evidence of bleeding; (4) not having been transfused in the previous 48 h at the moment of inclusion; (5) pretransfusion hemoglobin levels below 100 g/l; (6) hemodynamic stability (mean arterial pressure above 75 mmHg with no or low-dose vasoactive drugs); (7) controlled mechanical ventilation (patients sedated and fully adapted to the ventilator); (8) PaO2/FiO2 ratio > 250; and (9) temperature < 38 °C. Unstable patients (frequent changes in arterial pressure, inspired oxygen concentration or ventilatory regimen) and those requiring urgent surgery within the next few hours were excluded.
Study strategy
Monitoring of brain tissue oxygen pressure
To monitor cerebral oxygen pressure we used the LICOX® IMC System developed by GMS (Kiel-Mielkendorf, Germany). The system consists of a precalibrated polarographic Clark-type electrode [11, 12], an introducer assembly and a monitor to measure and display the PtiO2. Before and after inserting the probe, the proper location in the cerebral white matter was assessed by head computed tomography. The probe was inserted by the neurosurgeon through a triple-lumen transcranial bolt, allowing simultaneous introduction of the oxygen-sensing catheter, a fiberoptic ICP catheter and a temperature sensor directly into the brain parenchyma. The distal tip of the catheter was placed into the uninjured frontal white matter, to a depth of 25–30 mm below the dura; Following the insertion of the oxygen-sensing catheter, approximately 120 min were allowed for stabilization of the sensor before measurements were started. We want to highlight that, in all cases, the Licox catheter had previously been inserted for indications other than inclusion in this study, the patient's relatives being asked for their informed consent by the doctor in charge.
Monitoring of other variables
Blood pressure recording was obtained with a radial artery fluid-coupled system. End-tidal carbon dioxide (EtCO2) and oxygen saturation were continuously monitored with capnigraphy and pulse oximetry.
All patients were sedated with an infusion of midazolam and morphine. Additionally, a vecuronium infusion was used when proper synchronization with the ventilator was not possible. During the first 6 h of monitoring, all physiologic variables were closely observed, and changes in ventilatory setting, EtCO2, FiO2, levels of sedation and analgesia, and the infusion of fluid and drugs were minimized. During the study, ventilation was adjusted to maintain normal EtCO2 values and oxygen saturation above 96%.
After baseline measurements, 1 or 2 units of packed erythrocytes (1 unit approximately 270 ml) were transfused over a period of 120 min, depending on the baseline hemoglobin level and the patient's clinical status. For the purposes of our study, a transfusion episode was defined as the transfusion of 1 or 2 units (when 2 units were transfused, both units had been stored for a similar period of time). Patients received leukocyte-depleted packed erythrocytes that had been depleted of the buffy coat. In all of the cases, the additive solution consisted of saline, glucose, mannitol, and an anticoagulant preservative such as citrate–phosphate–dextrose. All transfusions were elective. The indication of transfusion was made by the doctor responsible for the patient and was independent of the purposes of this study. In our center, the request for informed consent for elective transfusion is routine. The major criterion for transfusion was pretransfusion hemoglobin below 100 g/l, always with careful assessment of the patient's clinical status.
Study measurements
Ten sets of data were collected in each transfusion: one at baseline (pretransfusion), and nine post-transfusion sets (end of transfusion and 1, 2, 3, 4, 5, 6, 12 and 24 h post-transfusion). Arterial blood lactate levels and PaO2/FiO2 ratio were measured only twice: at baseline and 24 h post-transfusion. Hemoglobin levels were measured at baseline and at hours 3 and 24 post-transfusion. PtiO2, CPP, ICP, MAP, EtCO2, were measured once in each set. All the data were collected in a prospective fashion.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of the distribution of continuous variables. All data are presented as mean ± SD unless otherwise stated. The changes of physiological variables to different times were evaluated by means of multiple analysis of variance (MANOVA). If significant changes were found, a paired t-test with Bonferroni correction was used for evaluating differences between the baseline value and the consecutive time points.
The effect of blood transfusion on both PtiO2 groups (< 15 vs. ≥ 15 mmHg) was obtained using MANOVA with repeated measures, with the PtiO2 (< 15 vs. ≥ 15 mmHg ) as the between-subject variable and time point (baseline, end of RBCT, and 1, 2, 3, 4, 5, 6, 12 and 24 h after RBCT) as the within-subject variables. Simple and multiple linear regressions were used to examine the association between the dependent variable (changes in PtiO2) and baseline individual independent variables. Comparisons of categorical and continuous variables by group (PtiO2 < 15 vs. ≥ 15 mmHg) were done using chi-squared tests and the independent sample t-tests (two-tailed), respectively.
All analyses were calculated with a statistical software (SPSS 13.0; SPSS, Chicago, IL) using a 5% significance level.
Results
Sixty patients were included. Table 1 shows patients' characteristics. Ninety-one units of packed red blood cells were transfused. Forty-seven patients (78.3%) showed an increment in PtiO2 at hour 3 post-transfusion (maximum peak); however, only 50% of them maintained this increase at hour 24. Similarly, significant increments in hemoglobin levels were observed at hours 3 (p < 0.01) and 24 (p < 0.01). Conversely, no changes occurred in lactate levels and PaO2/FiO2 ratio with regard to baseline values (Table 2).
Global effects of blood transfusion
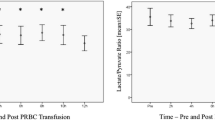
Before transfusion, PtiO2 was 24.4 ± 9.1 mmHg. After transfusion, a significant increase in PtiO2 was observed (end of transfusion and hours 1–6) (Table 3) that remained above baseline values during the whole study. CPP also increased in a similar way. The point of maximum increment in PtiO2 was at hour 3 (26.2% of increment; p = 0.0001). Such increment was independent from concomitant increments in CPP (7.3%; correlation coefficient 0.06; p = 0.66) and hemoglobin (19.1%; correlation coefficient 0.29; p = 0.06) (Fig. 1). Additionally, no correlation was found either between the increments observed in PtiO2 and CPP in any of the other intervals, or between PtiO2 and hemoglobin when assessed at hour 24 (data not shown). Patients transfused with one or two blood units had similar increments in PtiO2 (p = 0.37) (hour 3). EtCO2 and temperature did not show significant variations during the whole study.
Histogram illustrating percent mean changes in physiologic variables 3 h and 24 h after transfusion. RBCT red blood cell concentrates transfusion; Hb hemoglobin; PtiO 2 brain tissue oxygen pressure; CPP cerebral perfusion pressure. No correlation was found between PtiO2, hemoglobin, and CPP increments
Influence of baseline values on PtiO2 variations resulting from blood transfusion
The individual absolute change in PtiO2 at hour 3 resulting from blood transfusion was not significantly correlated with pretransfusion values of PtiO2, CPP, hemoglobin, transfused volume (1 or 2 units), length of storage of transfused units, age, sex, days passed since admittance to the ICU to the first transfusion (days before transfusion) or severity scorings (GCS, APACHE, ISS, TCDB). The relative change in PtiO2 was only significantly correlated with baseline PtiO2 (Fig. 2).
Since the relative increase in PtiO2 at hour 3 was related only with baseline PtiO2, we classified patients according to their baseline PtiO2. Patients with high or low initial levels of PtiO2 (< 15 vs. ≥ 15 mmHg) showed an increase in PtiO2 at hours 3 and 24 with regard to baseline. However, although both groups significantly increased PtiO2, only those patients with initial PtiO2 < 15 mmHg maintained PtiO2 significantly increased after 24 h post-transfusion (Table 4). At hour 3, every patient (n = 9) with basal PtiO2 < 15 mmHg increased PtiO2 while only 74.5% of patients with basal PtiO2 ≥ 15 did (p<0.01) (Table 4). Interestingly, after transfusion, every patient (N.9) with PtiO2 < 15 mmHg (hypoxia range) remained in normoxia for nearly the whole study interval (Fig. 3). No differences were present between both groups regarding baseline characteristics (Table 5), except for the number of days before transfusion. Therefore, a further ANOVA analysis was carried out using the relative number of patients who increased PtiO2 at hour 3 in each group as the dependent variable, and the patient's group and the number of days before transfusion as independent variables. In this analysis, the effect of this last variable on PtiO2 increments disappeared (p<0.154), thus leaving baseline PtiO2 (< 15 vs. ≥ 15 mmHg) as the only variable with a demonstrated impact on further PtiO2 increments.
Discussion
The major finding of our work is that blood transfusion is associated with a variable increment in cerebral oxygenation in patients with severe traumatic brain injury. This PtiO2 increment is not related with parallel increments of CPP and hemoglobin and is best related to low levels of PtiO2 before a blood transfusion. In addition, patient-related variables such as age, gender, severity score and days before transfusion did not influence the increase in PtiO2.
Transfusion has been recently confirmed as a strong and independent predictor of poor outcome, associated with an increase in the rates of mortality and morbidity, in the neurological critical care population. In a prospective cohort study including more than 100,000 consecutive patients followed over a period of 10 years, the history of blood transfusion was found to be a independent risk factor for fatal subarachnoid hemorrhage (RR 2.04 [1.26–3.33]) [8] and was significantly associated with increased mortality from ischemic stroke (RR 1.63 [1.18–2.23]) and intracerebral hemorrhage (RR 2.16[1.42–3.27]) [10]. Smith et al. [9] recently reported an increased risk of vasospasm and poor outcome in patients who received intraoperative blood transfusion. Therefore, before subjecting a patient to the inherent risks of blood transfusion it is a major concern to demonstrate the role of transfusion in increasing tissue oxygenation.
Our findings show that blood transfusion is associated with a variable increase in cerebral oxygenation. In 78.3% of patients PtiO2 increased within the interval of maximum increment (hour 3) although in only 50% of them did PtiO2 remain above baseline levels 24 h after transfusion (Table 2). The variable effect of transfusion has previously been reported [7, 13]. Smith et al. [13] demonstrated that erythrocytes transfusion is associated with an early increment of PtiO2 (1 h post-transfusion) in most patients (74%) with subarachnoid hemorrhage or severe TBI. Casutt et al. [7] studied the effects of transfusion in 77 cardiac surgery patients, showing that oxygen delivery increased without an increase in oxygen consumption. The influence of erythrocyte transfusion on skeletal muscle oxygen tension was studied in 51 patients undergoing cardiac surgery [15]. No changes were observed either in muscular PtiO2 or in oxygen consumption after the transfusion of 1–2 units of erythrocytes. Conversely, in an experimental animal model of rats with brain injury [16], administration of perfluorocarbon increased PtiO2 in a dose-dependent manner compared with a control group treated with saline solution.
This failure of blood transfusion to consistently increase PtiO2 is not well understood. Several factors may explain the absence of a beneficial effect of blood transfusion. First, it could be that cerebral oxygen consumption was not dependent on oxygen supply. Second, prolonged storage may interfere with the ability of erythrocytes to transport and unload oxygen. Third, transfusion of erythrocytes is associated with an increase in hematocrit, which contributes to blood viscosity, and clinical studies have demonstrated an inverse relationship between hematocrit and cerebral blood flow. A high hematocrit level may potentially decrease cerebral blood flow and increase the risk of ischemia [17].
Erythrocyte transfusion caused an increase in PtiO2, CPP and hemoglobin levels (Tables 2 and 3). However, these increases were not correlated during the whole study period. The relationship between PtiO2 and CPP is controversial. Several studies have shown that PtiO2 depends on CPP in ischemic areas [18], and even that a correlation exists between CPP and PtiO2 [19]. In other authors' opinion, even marked increases in CPP do not increase oxygenation [20]. In a recent study similar to ours [13], no significant association was observed between the changes in PtiO2 and those in CPP and transfused volume.
No relationship was found between the different increments in PtiO2 and hemoglobin levels, as assessed at hours 3 and 24. On the contrary, Smith et al. [13] found that PtiO2 increments were only associated with a significant mean increase in hemoglobin. There are important differences between both articles. We studied the effects of transfusion on PtiO2 during a prolonged time (24 h), while Smith et al. restricted their study to just the first hour post-transfusion. Since 2,3-DPG levels take several hours to recover to baseline [14], it is possible that the greatest benefits are not appreciable until several hours have passed after transfusion. However, when assessing the relationship between PtiO2 and hemoglobin levels at 24 h, by when 2,3-DPG levels had recovered more than 50%, no correlation was observed either. These results confirm those of Cassutt et al. [7], who reported that the increase in oxygen consumption after erythrocyte transfusion did not correlate with an increase in hemoglobin levels.
At present, it is not possible to predict which patients will respond to a blood transfusion with an increase in PtiO2, since this has not been prospectively investigated. In order to answer this question, we carried out two additional investigations. In a first analysis, the changes observed in PtiO2 at hour 3 (the time of peak increment) resulting from blood transfusion were related to a variety of baseline variables by using simple linear regression analyses, including PtiO2, CPP, hemoglobin, age, gender, days before transfusion and severity scoring. The individual relative increment in PtiO2 showed an inverse relationship only with baseline PtiO2 (Fig. 2). No other relationship was found between the absolute or relative increment in PtiO2 and the baseline variables. Again, these findings are in accordance with those of Cassutt et al. [7]. In 77 patients undergoing cardiac surgery, the individual increase in the consumption index was inversely related to the oxygen consumption index before transfusion (p<0.001). A recently published prospective randomized trial in humans with a reproducible oxygen-dependent deficit demonstrated that transfusion of erythrocytes is efficacious for reversing the effects of acute isovolemic anemia [21]. This study suggested that physiologic transfusion triggers will progressively replace arbitrary hemoglobin-based transfusion triggers [22]. It is possible that determining baseline PtiO2 allows us to better predict the individual effect of a blood transfusion in anemic neurocritical patients.
Some studies have suggested an inverse relationship between PtiO2 and unfavorable clinical outcome. Values below 15 mmHg have proved to be an independent predictor of unfavorable outcome and death [11, 23]. Since in our study the relative increment in PtiO2 was related to baseline PtiO2, a second analysis was carried out. A cut-off of 15 mmHg was used to classify patients according to baseline levels of PtiO2 (low and high PtiO2). At hour 3, every patient (n = 9, 100%) with a PtiO2 < 15 mmHg increased PtiO2 above 15 mmHg. (Table 4, Fig. 3). On the contrary, only 74.5% of the patients with PtiO2 ≥ 15 mmHg presented additional increments in PtiO2 (p<0.01). No baseline differences were found between patients with low or high baseline PtiO2 (Table 5). The multivariate analysis demonstrated that PtiO2 baseline level < 15 mmHg directly correlates with the percentage of patients increasing PtiO2 at hour 3 (p<0.001). Although these results are preliminary and need corroboration, the present study indicates that patients with anemia and PtiO2 < 15 mmHg (cerebral hypoxia) may improve their oxygenation to PtiO2 levels of ≥ 15 mmHg (cerebral normoxia) after blood transfusion.
We must point out that this study has limitations inherent to an observational design. (1) An observational study cannot establish causal relationships. (2) We cannot be sure whether the PtiO2 increment was an effect of colloids, CPP or a true red cell effect. Although there are no studies available designed to demonstrate the effect of colloids on PtiO2, it is known that physiological saline solution increases PtiO2 [16]; therefore, it is possible that the increment in PtiO2 was a result of the volume expansion effect caused by the transfusion rather than an effect of the erythrocytes themselves, even though no significant correlation could be demonstrated. (3) Ideally, we should have determined cerebral blood flow and oxygen consumption, since demonstrating an increase in PtiO2 after blood transfusion does not mean a parallel increment in cerebral oxygen consumption. (4) Since PtiO2 monitoring is a regional measurement of brain oxygenation, the variations in PtiO2 do not necessarily reflect the level of oxygenation in other brain areas, including the site of injury; rather, they reflect how systemic factors may influence global variations in cerebral oxygenation. (5) Lastly, the sample in our study is relatively small. However, accepting an error alpha of 0.05 and an error beta of 0.20 in a unilateral contrast, and assuming a standard deviation of 10, only 25 individuals would be required to detect a difference equal or superior to 5 units in the changes of PtiO2 (from 10 mmHg, the lowest value in our sample, to 15 mmHg, as considered the lowest limit of normoxia).
It is important to underline that our findings are preliminary and need to be corroborated. Thus, whether brain oxygen values can be used to guide transfusion practices in neurocritical patients or how transfusion increases brain oxygen are questions that remain unanswered
In summary, erythrocyte transfusion is associated with a variable and prolonged increase in cerebral oxygenation in patients with severe TBI, even though the mechanism by which PtiO2 increases is not yet well established. Low baseline PtiO2 levels could define those patients who may benefit the most from erythrocyte transfusion. These data may be crucial in demonstrating the efficacy of blood transfusion and they might help in defining a threshold from which blood transfusion would yield the highest benefits in patients with severe TBI, and therefore be indicated. Nevertheless, we are aware that further research on the potential effects of blood transfusion on tissue oxygenation is still needed.
Abbreviations
- CPP:
-
cerebral perfusion pressure
- EtCO2 :
-
end-tidal CO2,
- FiO2 :
-
oxygen inspired fraction
- ICP:
-
Intracranial pressure
- PaO2 :
-
oxygen arterial pressure
- PtiO2 :
-
Brain tissue oxygen pressure
- TBI:
-
traumatic brain injury
- StO2 :
-
peripheral saturation assessed by pulse oximetry
References
Walsh TS, Lee RJ, Maciver CR, Garrioch M, Mackirdy F, Binning AR, Cole S, McClelland DB (2006) Anemia during and at discharge from intensive care: the impact of restrictive blood transfusion practice. Intensive Care Med 32:100–109
Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, ABC (Anemia and Blood Transfusion in Critical Care Investigators) (2002) Anemia and blood transfusion in critically ill patients. JAMA 288:1499–1507
Leal-Noval SR, Marquez-Vacaro JA, Garcia-Curiel A, Camacho-Larana P, Rincon-Ferrari MD, Ordoñez-Fernandez A, Flores-Cordero JM, Loscertales-Abril J (2000) Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med 28:935–940
Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM (2003) Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma 54:898–890
Fernandes CJ, Akamine N, De Marco FV, de Sousa JA, Lagudis S, Knobel E (2001) Erythrocytes transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care 5:362–367
Marik PE, Sibbald WJ (1993) Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 269:3024–3029
Casutt M, Seifert B, Pasch T, Schmid E, Turina M, Spahn D. (1999) Factors influecing the individual effects of blood transfusion on oxygen delivery and oxygen consumption. Crit Care Med 27:2194–2200
Yamada S, Koizumi A, Iso H, Wada Y, Watanabe Y, Date C, Yamamoto A, Kikushi S, Inaba Y, Toyoshima H, Kondo T, Tamokoshi A and JACC Study Group (2003) Risk factors for fatal subarachnoid hemorrhage: the Japan Collaborative Cohort Study. Stroke 34:2781–2787
Smith MJ, Le Roux PD, Eliott JP, Winn HR (2004) Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg 101:1–7
Yamada S, Koizumi A, Iso H, Wada Y, Watanabe Y, Date C, Yamamoto A, Kikushi S, Inaba Y, Kondo T, Toyoshima H, Tamokoshi A and JACC Study Group (2005) History of blood transfusion before 1990 is a risk factor for stroke and cardiovascular diseases: the Japan collaborative cohort study (JACC study).Cerebrovasc Dis 20:164–171
Van den Brink WA, van Santbrink H, Steyerberg EW, Avezaat CJ, Suazo JA, Hogesteeger C, Jansen WJ, Kloos LM, Vermeulen J, Maas AI (2000) Brain oxygen tension in severe head injury. Neurosurgery 46:868–876
Dings J, Meixensberger J, Jager A, Roosen K (1998) Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 43:1082–1095
Smith MJ, Stiefel MF, Magge S, Frangos S, Blom S, Gracias V, Le Roux PD (2005) Packed erythrocytes transfusion increases local cerebral oxygenation. Crit Care Med 33:1104–1108
Ho J, Sibbald WJ, Chin-Y EE (2003) Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med 31:S687–S697
Suttners S, Piper SN, Kumle B, Lang K, Rohm KD, Isgro F, Boldt J (2004) The influence of allogeneic blood cell transfusion compared with 100% oxygen ventilation on systemic oxygen transport and skeletal muscle oxygen tension after cardiac surgery. Anesth Analg 99:2–11
Daugherty WP, Levasseur JE, Sun D, Rockswold GL, Bullock MR (2004) Perfluorocarbon emulsion improves cerebral oxygenation and mitochondrial function after fluid percussion brain injury in rats. Neurosurgery 54:1223–1230
Pendem S, Rana S, Manno E, Gagic O (2006) A review of red cell transfusion in the neurological intensive care unit. Neurocrit Care 4:63–67
Lang EW, Czosnyka M, Mehdorn M (2003) Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit Care Med 31:267–271
Marín-Caballos AJ, Murillo-Cabezas F, Cayuela-Domínguez A, Domínguez-Roldan JM, Rincón-Ferrari MD, Valencia-Anguita J, Flores-Cordero JM, Muñoz-Sánchez MA (2005) Cerebral perfusion pressure and risk of brain hypoxia in severe head injury: a prospective observational study. Crit Care 9(6):670–676
Sahuquillo J, Amoros S, Santos A, Poca MA, Panzardo H, Dominguez L, Pedraza S (2000) Does an increase in cerebral perfusion always mean a better oxygenated brain? A study in head-injured patients. Acta Neurochir Suppl 76:457–462
Weiskopf RB, Feiner J, Hopt H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P (2006) Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology 104:911–920
Spahn D, Madjdpour C (2006) Physiologic transfusion triggers. Anesthesiology 104:905–906
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS (1998) Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med 26:1576–1581
Acknowledgements
Supported by Spanish Government funds (Fondo de Investigación Sanitaria: Proyecto de Investigación PI 040296; Convenio específico de colaboración entre el Instituto de Salud Carlos III y la comunidad autónoma andaluza, fundación “Progreso y Salud”. BOE 31, resolución 1907, año 2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leal-Noval, S.R., Rincón-Ferrari, M.D., Marin-Niebla, A. et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med 32, 1733–1740 (2006). https://doi.org/10.1007/s00134-006-0376-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0376-2