Abstract
Periprosthetic fractures after massive endoprosthetic reconstructions pose a reconstructive challenge and jeopardize limb preservation. Compressive osseointegration technology offers the promise of relative ease of prosthetic revision, since fixation is achieved by means of a short intramedullary device. We retrospectively reviewed the charts of 221 patients who had Compress® devices implanted in two centers between December, 1996 and December, 2008. The mean followup was 50 months (range, 1–123 months). Six patients (2.7%) sustained periprosthetic fractures and eight (3.6%) had nonperiprosthetic ipsilateral limb fractures occurring from 4 to 79 months postoperatively. All periprosthetic fractures occurred in patients with distal femoral implants (6/154, 3.9%). Surgery was performed in all six patients with periprosthetic femur fractures and for one with a nonperiprosthetic patellar fracture. The osseointegrated interface was radiographically stable in all 14 cases. All six patients with periprosthetic fracture underwent limb salvage procedures. Five patients had prosthetic revision; one patient who had internal fixation of the fracture ultimately underwent amputation for persistent infection. Periprosthetic fractures involving Compress® fixation occur infrequently and most can be treated successfully with further surgery. When implant revision is needed, the bone preserved by virtue of using a shorter intramedullary Compress® device as compared to conventional stems, allows for less complex surgery, making limb preservation more likely.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Introduction
Given the increasing number of primary and revision arthroplasty cases performed, the prevalence of uncemented devices, the improvements in implant survivorship, and an active but aging patient population with attendant comorbidities such as osteoporosis, periprosthetic fractures are an increasingly common complication [4]. The incidence of periprosthetic femoral fractures around total hip and knee replacements has been estimated at 4.1% and 2.8%, respectively [3, 16, 17, 38]. A recent review concluded, however, “There is little information about the overall incidence (the risk) of periprosthetic fracture in a broader perspective, taking, for example, time since operation into account” [24]. Furthermore, treatment algorithms are often quite complex [26], and little has been published regarding treatment outcomes.
Comparatively less is known about the incidence and results of periprosthetic fractures around massive endoprosthetic implants typically used for limb salvage performed for tumors. Although a recent report cited distal femoral and proximal tibial periprosthetic fracture rates of 0.9% and 2%, respectively [20], the literature provides little guidance regarding management and expected outcome of such fractures. For conventional cemented and uncemented devices, revision is complicated by excessive stem length and consequent sacrifice of bone stock in patients who frequently have high activity demands and a long life expectancy.
Compressive osseointegration technology was devised to provide stable fixation of endoprostheses by way of a novel spring-loaded system anchored by a short intramedullary device [2, 5, 9, 11, 23, 30]. Some concern existed that the Compress® implant would potentially be subject to an increased risk of periprosthetic fracture particularly at the transverse pin placement sites. Furthermore, it was thought that any torque load sufficient to cause a periprosthetic fracture would also result in disruption of the bone-prosthetic interface. Initial prosthetic survivorship results nonetheless have been encouraging when distal femoral Compress® implants were compared with conventional cemented stems [5]. As our experience with the Compress® device has developed, we have noted that the design allows for relatively straightforward revision in cases of infection or periprosthetic fracture [30].
Our purposes were to determine: (1) the frequency of ipsilateral limb fractures associated with Compress® implants; (2) whether they were periprosthetic or nonperiprosthetic; (3) time to fracture; (4) how they were treated; and (5) the effect of these fractures on prosthetic retention, maintenance of limb salvage, and ambulatory status.
Materials and Methods
We retrospectively reviewed the records of 221 patients who had Compress® devices implanted between December 1996 and December 2008 for patients with the following indications: primary oncology (165); revision oncology (33); revision arthroplasty (18); and post-traumatic reconstruction (five). The anatomic locations were as follows: distal femoral (154), proximal tibial (38), proximal femoral (23), distal humeral (four), and proximal humeral (two). All ipsilateral limb fractures were recorded, and classified as periprosthetic or nonperiprosthetic (defined as fractures not adjacent to the Compress® implant). The minimum followup for patients with periprosthetic fractures after fracture treatment was 14 months (median, 62 months; range, 14–94 months) and the minimum followup for nonperiprosthetic fractures was 4 months (median, 24 months; range, 4–89 months). No patients were lost to followup. We obtained prior Institutional Review Board approval.
Previously published methods of Compress® surgery were followed [30]. For any given primary or revision indication, an osteotomy through bone of normal quality was made perpendicular to the longitudinal axis of the bone. Triple reamers were utilized to ensure the diameter of the centering sleeve of the device was at least 2 mm larger than that of the anchor plug. Transverse pins measuring 4 mm longer than the bone diameter were implanted through bone of normal quality. Hydroxyapatite-coated spindles of short length and large diameter with 800 lbs. of force are now frequently chosen for the femur, while short, small, 600 lb. hydroxyapatite-coated spindles are selected for tibial cases; anti-rotation pins are generally not utilized. Care was taken to achieve a tight fit between the centering sleeve and the endosteal surface.
To minimize the risk of periprosthetic fracture or spindle failure through application of rotational torques, patients with femoral and tibial reconstructions were maintained at a strictly nonweightbearing gait protocol for six and twelve weeks, respectively. Thereafter, partial weightbearing was advanced 25% per week. Gentle active and active-assisted range of motion and strengthening (quadriceps sets and straight leg raising) exercises were begun immediately after surgery. Calcium and vitamin D were also routinely prescribed. Recreational pursuits more vigorous than hiking, bicycling, and swimming are strongly discouraged; high or repetitive impact activities and contact sports are not allowed [30].
Plain radiographs were obtained before discharge from the hospital, as well as at 2 and 6 weeks after surgery. Clinical and radiographic examinations were generally performed at 3-month intervals for the first 2 years after surgery, and at 6-month intervals thereafter. From the medical records we obtained the following demographic data: gender, age, surgical indications (primary or revision; tumor, arthroplasty, or trauma), and whether chemotherapy or radiation therapy was administered. Radiographs were examined to identify technical errors, evidence of implant breakage, or disruption of the bone-prosthetic interface. From the charts we recorded time to fracture, mechanism of fracture, fracture management, and outcome with respect to prosthetic retention, limb preservation, and ambulatory status.
Results
Fourteen of the 221 patients (6.3%) had ipsilateral limb fractures. Six of these (2.7%) were periprosthetic and eight (3.6%) were nonperiprosthetic.
Among those with periprosthetic fractures, there were two male and four female patients with a median age of 24.5 years (range, 13-32 years). Five patients underwent Compress® reconstructions for tumors; one cancer patient underwent revision of a failed conventional stemmed device. Three patients received chemotherapy after Compress® surgery; none received radiation. Median time to fracture was 6 months (range, 2–20 months). All periprosthetic fractures occurred in association with distal femoral implants (6/154, 3.9%). Surgery was necessitated in all patients with periprosthetic fractures. Although revision was necessitated in one patient who sustained a fracture at the site of anti-rotation pin insertion (Fig. 1), four revisions were undertaken for the more common fracture pattern that occurred above the anchor plug (Fig. 2). One patient underwent open reduction and internal fixation (ORIF) (Fig. 3). The osseointegration site was intact in all cases. There were no instances of implant fracture. At last followup five patients retained their prostheses without further surgery and were walking without an assistive device; one patient ultimately underwent amputation for persistent infection (Table 1).
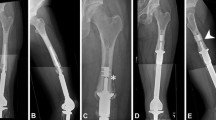
(A) Preoperative and (B) postrevision radiographs of a 32 year-old man who sustained a periprosthetic fracture within two months after surgery while bearing full weight, contrary to instructions. (A) The cortical bone splintered where anti-rotation pins had been inserted into the cortex, just above the osseointegration site; intraoperative findings noted that the compression force had not been lost. (B) Revised implant after resection of an additional 4 cm of bone.
(A) Preoperative anteroposterior and (B) 5.9 year postoperative lateral radiographs show a 17-year-old woman with a history of distal femoral osteosarcoma who sustained a displaced periprosthetic femoral shaft fracture 1.7 years after treatment with chemotherapy and Compress® reconstruction. (A) Despite the fracture, the osseointegrated interface remained intact. (B) Revision was accomplished in a straightforward manner by resection of a minimal amount of bone and reimplantation of a Compress® device.
(A) Preoperative and (B) postoperative anteroposterior radiographs of a 16 year-old woman with a periprosthetic femoral fracture occurring at an area of cortical thinning where an uncemented stem had previously been present. (A) The Compress® prosthetic-bone interface is stable. (B) Open reduction and internal fixation was undertaken without the need for prosthetic revision.
Among those with minor ipsilateral nonperiprosthetic fractures, there were five male and three female patients with a median age of 14 years (range, 10-29 years). Seven patients underwent Compress® reconstructions for tumors; one cancer patient underwent revision of a failed Compress® device. Except for this patient, all patients received chemotherapy after Compress® surgery; one received adjuvant radiation. Median time to fracture was 8.5 months (range, 4–79 months). Minor fractures occurred in five distal femoral (5/154, 3.2%) and three proximal tibial (3/38, 7.9%) cases. Fractures in this group included distal tibial (four), patellar (two), proximal tibial (one), and proximal fibular (one) locations. Median time to fracture was 7 months (range, 4–79 months). Only one patient, with a patellar fracture, required open reduction and internal fixation (Fig. 4). The remaining patients were treated nonoperatively. At last followup all prostheses were retained, and patients were able to walk without the need for assistive device. Despite forces sufficient to cause fracture, there were no cases of device breakage, and the compressive osseointegration interface remained intact in all cases (Table 2).
(A) Preoperative and (B) 2.4 year postoperative lateral radiographs of an 18-year-old woman with a history of distal femoral sarcoma who sustained a displaced patellar fracture 3.8 years after treatment with chemotherapy, Compress® reconstruction, and radiation therapy are shown. (A) Despite a fall from a trampoline, the bone-prosthetic interfaced remained stable. (B) Healed fracture despite prior radiation is shown.
Discussion
We undertook this review to better understand the frequency, location, timing, and management of ipsilateral limb fractures associated with compressive osseointegration reconstructions. The effect of such fractures on prosthetic retention, limb preservation, and ambulatory status was also studied.
Limitations of this study include its size, length of followup, and focus on one type of endoprosthesis. The limited size and followup make it difficult to draw definitive conclusions regarding the time frame for fractures to occur. Experience with the Compress® device reported here is not directly applicable to periprosthetic fractures in arthroplasty patients, or even in the majority of tumor patients, who continue to receive conventional stemmed implants. Generalization of these results to centers less experienced in the use of compressive osseointegration techniques may not be immediately possible. Furthermore, the study population described here consists largely of young tumor patients, whereas the Compress® device is being increasingly used for revision arthroplasty patients, for whom advanced age and osteoporosis may well signal a higher periprosthetic fracture risk. Another limitation is the lack of firm data regarding risk analysis of periprosthetic fracture per patient-year [24]. However, the paper reports an experience of more than 200 patients treated over a 12-year period by four surgeons at two major sarcoma centers. In addition, the data would seem to confirm the acceptable risk profile of compressive osseointegration technology for endoprosthetic fixation with respect to the specific issues of periprosthetic fracture incidence, management, and prosthetic retention.
The most robust data regarding periprosthetic fractures from the arthroplasty literature deal with femoral fractures after total hip replacement, for which the 10-year probability of fracture has been estimated to be 0.64% [24]. The annual incidence is thought to vary between 0.045% and 0.13%, with a tendency for the incidence to increase over time [24]. Treatment is often complex [26], and the seriousness of this complication is highlighted by the 1-year mortality rate for such patients, which in one study was 12% for those undergoing revision arthroplasty and 33% for those undergoing ORIF [6].
Comparable information regarding incidence, treatment, and outcomes for patients with periprosthetic fractures after megaprosthetic reconstructions, most often performed for tumors, is lacking. Most large series of intermediate to long-term results of endoprosthetic implants highlight implant survivorship, but failure due to periprosthetic fracture is often not specifically commented upon [7, 8, 12–15, 18, 21, 25, 32, 33, 36, 37]. Although Inglis and Walker reported a periprosthetic fracture rate of 37.5% of fixed hinge devices used to revise failed hinged implants [19], most recent papers commenting on periprosthetic fracture risk in primary tumor reconstructions using rotating hinge devices report rates of 0.3% to 6.1% (Table 3) [1, 10, 20, 22, 27–29, 31, 34, 35, 39]. Given the relatively low frequency of this complication, and the wide variety of implants reported upon, little has been described regarding optimal surgical management or treatment results of these fractures.
As compared to historical data regarding arthroplasty patients as well as cancer patients having conventional cemented and uncemented stems, we believe the Compress® device provides acceptable results in terms of the incidence of periprosthetic fractures in a generally young tumor population which is nonetheless subject to risk factors of osteoporosis (secondary to preoperative disuse and the effects of chemotherapy and radiation) and high activity demands. When a fracture does occur, Compress® technology offers the distinct advantage of comparatively straightforward revision, given the ease of extraction of the intramedullary portion of the device, and the minimal amount of bone (as little as 2 to 4 cm) that needs to be resected before implantation of a new device. Furthermore, short metaphyseal-epiphyseal fragments (43 mm or longer) remaining after fracture can still be salvaged with a short anchor plug, thus obviating the need for conversion to a total femoral replacement [30]. Although femoral fractures above the anchor plug can be expected to occur at any time in the patient’s life if sufficient force is applied, our finding that all periprosthetic fractures occurred within 2 years of surgery is of potential importance for predicting the risk of this complication, since the opposite is expected to be true for typical arthroplasties and megaprostheses [24]. This difference can be attributed to the cortical hypertrophy engendered by compressive osseointegration forces; as demonstrated by Avedian et al. [2], the Compress® device provides, stability and bone growth at the prosthetic interface over the first 6 to 12 months, effectively sealing the endosteal canal to particulate debris [23, 30]. By contrast, stress shielding and osteolysis are expected to be ever-increasing problems for many tumor megaprosthetic stems, thereby increasing the risk for aseptic loosening and periprosthetic fracture with time. Finally, we observed no instances of mechanical breakage of the Compress® device, a finding that should be considered when comparing conventional endoprosthetic devices, for which implant fracture has been reported to be as high as 10% [14]. Although case-matched cohort studies are of some utility in comparing compressive osseointegration technology to standard stem fixation [5], long term prospective studies are desirable in order to elucidate this and other complications before any particular reconstructive approach can be definitively endorsed.
References
Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795.
Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53.
Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30:183–189.
Berry DJ. Periprosthetic fractures associated with osteolysis: A Problem on the rise. J Arthroplasty. 2003;18:107–111.
Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress® implant. Int Orthop. 2006;30:465–472.
Bhattacharyya T, Chang D, Meigs JB, Estok DM, II, Malchau H. Mortality after periprosthetic fracture of the femur. J Bone Joint Surg Am. 2007;89:2658–2662.
Biau D, Faure F, Katashian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293.
Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KLM, Isaac, Malawer MM. Distal femur resection with endoprosthetic reconstruction: A long-term followup study. Clin Orthop Relat Res. 2002;400:225–235.
Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: Interface retrieval data. Orthopedics. 2000;23:707–712.
Capanna R, Morris HG, Campanacci D, Del Ben M, Campanacci M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br. 1994;76:178–186.
Cristofolini L, Bini SA, Toni A. In vitro testing of a novel limb salvage prosthesis for the distal femur. Clin Biomech. 1998;13:608–615.
Frink SJ, Rutledge J, Lewis VO, Lin PP, Yasko AW. Favorable long-term results of prosthetic arthroplasty of the knee for distal femur neoplasms. Clin Orthop Relat Res. 2005;438:65–70.
Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171.
Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: Root causes of failure. Clin Orthop Relat Res. 2005;438:71–79.
Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494.
Gruner A, Hockertz T, Reilmann H. Die periprothetische fraktur: Klassifikation, management,therapie. Unfallchirurg. 2004;107:35–49.
Haddad FS, Masri BA, Garbuz DS, Duncan CP. The prevention of periprosthetic fractures in total hip and knee arthroplasty. Orthop Clin North Am. 1999;30:191–207.
Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30:452–457.
Inglis AE, Walker PS. Revision of failed knee replacements using fixed-hinge devices. J Bone Joint Surg Br. 1991;73:757–761.
Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271.
Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon D-G. A rotating hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14:187–196.
Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115.
Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: Retrieval study of Compress® implants. Int Orthop. 2008;32:567–571.
Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury. 2007;38:651–654.
Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165.
Masri BA, Meek RMD, Duncan CP. Periprosthetic fractures: Evaluation and treatment. Clin Orthop Relat Res. 2004;420:80–95.
Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177.
Mittermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Revision of the Kotz type of tumour endoprosthesis for the lower limb. J Bone Joint Surg Br. 2002;84:401–406.
Morgan HD, Cizik AM, Leopold SS, Hawkins DS, Conrad EU, III. Survival of tumor megaprostheses replacements about the knee. Clin Orthop Relat Res. 2006;450:39–45.
O’Donnell RJ. Compressive osseointegration of modular endoprostheses. Curr Opin Orthop. 2007;18:590–603.
Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: Modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30:458–464.
Plötz W, Rechl H, Burgkart R, Messmer C, Schelter R, Hipp E, Gradinger R. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin Orthop Relat Res. 2002;405:207–215.
Roberts P, Chan D, Grimer RJ, Sneath RS, Scales JT. Prosthetic replacement of the distal femur for primary bone tumours. J Bone Joint Surg Br. 1991;73:762–769.
Torbert JT, Fox EJ, Hosalkar HS, Ogilvie CM, Lackman RD. Endoprosthetic reconstructions: Results of long-term followup of 139 patients. Clin Orthop Relat Res. 2005;438:51–59.
Unwin PS, Cannon SR, Grimer RJ, Kemp HBS, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13.
Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses: The first 218 cases. J Arthroplasty. 1993;8:259–268.
Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74.
Younger AS, Dunwoody I, Duncan CP. Periprosthetic hip and knee fractures: The scope of the problem. Instr Course Lect. 1998;47:251–256.
Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–250.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tyler, W.K., Healey, J.H., Morris, C.D. et al. Compress® Periprosthetic Fractures: Interface Stability and Ease of Revision. Clin Orthop Relat Res 467, 2800–2806 (2009). https://doi.org/10.1007/s11999-009-0946-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-0946-z