Abstract
Background
It is conceivable that overstimulation of chemo- and mechano-sensors in the Roux and common limbs by uncontrolled influx of undigested nutrients after Roux-en-Y gastric bypass surgery (RYGB) could lead to exaggerated satiety signaling via vagal afferents and contribute to body weight loss. Because previous clinical and preclinical studies using vagotomy came to different conclusions, the aim was to examine the effects of selective and histologically verified celiac branch vagotomy on reduced food intake and body weight loss induced by RYGB.
Methods
Male Sprague–Dawley rats underwent either RYGB + celiac branch vagotomy (RYGB/VgX, n = 15), RYGB + sham celiac branch vagotomy (RYGB/Sham VgX; n = 6), Sham RYGB + celiac branch vagotomy (Sham/VgX; n = 6), or sham RYGB + sham celiac branch vagotomy (Sham/Sham; n = 6), and body weight, body composition, and food choice were monitored for 3 months after intervention.
Results
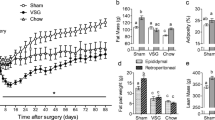
In rats with RYGB, histologically confirmed celiac branch vagotomy significantly moderated weight loss during the first 40 days after surgery, compared to either sham or failed vagotomy (P < 0.05). In contrast, celiac branch vagotomy slightly, but non-significantly, reduced body weight gain in sham RYGB rats compared to sham/sham rats. Furthermore, the significant food intake suppression during the first 32 days after RYGB (P < 0.05) was also moderated in rats with verified celiac branch vagotomy.
Conclusions
The results suggest that signals carried by vagal afferents from the mid and lower intestines contribute to the early RYGB-induced body weight loss and reduction of food intake.





Similar content being viewed by others
References
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–13.
Arnold M, Mura A, Langhans W, et al. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60.
Reidelberger RD, Hernandez J, Fritzsch B, et al. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–12.
Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80.
Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–46.
Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75.
Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–41.
Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8.
Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4.
Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res. 2005;1044:127–31.
Berthoud HR. Vagal and hormonal gut–brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20 Suppl 1:64–72.
Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–31.
Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–9.
Kissileff HR, Carretta JC, Geliebter A, et al. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R992–8.
Miranda A, Mickle A, Medda B, et al. Altered mechanosensitive properties of vagal afferent fibers innervating the stomach following gastric surgery in rats. Neuroscience. 2009;162:1299–306.
Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17.
Berthoud HR, Patterson LM, Neumann F, et al. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl). 1997;195:183–91.
Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–68.
Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–82.
Furnes MW, Stenstrom B, Tommeras K, et al. Feeding behavior in rats subjected to gastrectomy or gastric bypass surgery. Eur Surg Res. 2008;40:279–88.
Bjorklund P, Laurenius A, Een E, et al. Is the roux limb a determinant for meal size after gastric bypass surgery? Obes Surg. 2010;20:1408–14.
Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 Suppl 1:i2–5.
Hao Z, Zhao Z, Berthoud HR, et al. Development and verification of a mouse model for roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE. 2013;8:e52922.
Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253:R361–70.
Berthoud HR, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol. 1990;258:R523–30.
Shin AC, Zheng H, Townsend RL, et al. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obes Surg. 2013;23:531–40.
Bueter M, Lowenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–22.
Wang Y, Liu J. Combination of bypassing stomach and vagus dissection in high-fat diet-induced obese rats—a long-term investigation. Obes Surg. 2010;20:375–9.
Perathoner A, Weiss H, Santner W, et al. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg. 2009;19:412–7.
Raybould HE, Tache Y. Capsaicin-sensitive vagal afferent fibers and stimulation of gastric acid secretion in anesthetized rats. Eur J Pharmacol. 1989;167:237–43.
Kelly L, Morales S, Smith BK, et al. Capsaicin-treated rats permanently overingest low- but not high-concentration sucrose solutions. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1805–12.
Schwartz GJ, Salorio CF, Skoglund C, et al. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–9.
Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut–brain communication. Physiol Behav. 2011;105:106–19.
Berthoud HR, Kressel M, Raybould HE, et al. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl). 1995;191:203–12.
Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–60.
Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol. 1998;274:R1390–6.
Acknowledgments
We thank Katie Bailey for editorial help. This work was supported by National Institutes of Health Grants DK 47348 (HRB), DK 071082 (HRB), and DK 068036 (JY).
Conflict of Interest
Except for grant support from the NIH, none of the authors declares a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, Z., Townsend, R.L., Mumphrey, M.B. et al. Vagal Innervation of Intestine Contributes to Weight Loss After Roux-en-Y Gastric Bypass Surgery in Rats. OBES SURG 24, 2145–2151 (2014). https://doi.org/10.1007/s11695-014-1338-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1338-3