Abstract
Purpose
The purpose of the research is to investigate the application of bagasse fly ash, a sugar industry solid waste for the synthesis of zeolites and their behavior for the sorption of p-nitrophenol (p-NP).
Methods
Zeolitic materials were prepared from bagasse fly ash using alkaline hydrothermal (CZBFA) and fusion (FZBFA) treatment. Comparative batch sorption studies of prepared zeolitic material and virgin material were undertaken to determine their capacities for removal of p-nitrophenol.
Results
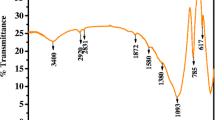
PXRD patterns revealed that zeolite P and analcime were the dominant contents of synthesized zeolitic material. Chemical composition, morphology, and crystalline nature of CZBFA and FZBFA were characterized by XRF, FTIR, and SEM. The Langmuir, Freundlich, Dubinin Redushkwich, and Temkin sorption isotherms were applied to compare the sorption nature and capacity of synthesized CZBFA and FZBFA with virgin BFA. For each sorbent-p-NP system, a pseudo-second-order kinetic model described the sorption kinetics accurately. The thermodynamics of the p-NP-sorbent systems exhibit an exothermic sorption process. Intraparticle diffusion model shows that the sorption rate was controlled by film diffusion followed by pore diffusion. Regeneration of sorbents was carried out by desorption studies with HCl, NaOH, and SDS detergent. The column studies were performed for the practical utility of sorbents, and breakthrough curve were obtained, which exhibit higher sorption capacity than batch method.
Conclusion
The sorption capacities of the synthesized zeolites had improved sorption capacities for the sequestration of p-NP and can be utilized as low-cost sorbents for treatment of p-nitrophenolic wastewater.




Similar content being viewed by others
References
Ahmaruzzaman M, Laxmi GS (2010) Activated tea waste as a potential low-cost adsorbent for the removal of p-nitrophenol from wastewater. J Chem Eng Data 55:4614–4623
Ahmaruzzaman M, Sharma DK (2005) Adsorption of phenols from wastewater. J Colloid Interface Sci 287:14–24
Anirudhan TS, Sreekumari SS, Brigle CD (2009) Removal of phenols from water and petroleum industry refinery effluents by activated carbon obtained from coconut coir pith. Adsorption 15:439–451
Basu JK, Dutta S, Ghar RN (2001) Studies on adsorption of p-nitrophenol on charred saw-dust. Sep Purif Technol 21:227–235
Bhatnagar A (2007) Removal of bromophenol from water using industrial wastes as low cost adsorbents. J Hazard Mater B139:93–102
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Crini G, Morin N, Rouland JC, Janus L, Morcellet M, Bertini S (2002) Adsorption de beta-napthol sur des gels de cyclodextrine-carboxym-ethyl-cellulose reticules. Eur Polym J 38:1095–1103
Dabrowski A, Podkoscielny P, Hubicki M, Barczak M (2005) Adsorption of phenolic compounds by activated carbons a critical review. Chemosphere 58:1049–1070
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Acad Sci USSR Phys Chem Sec 55:331–337
Fierro V, Torné-Fernández V, Montané D, Celzard A (2008) Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater 111:276–284
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Gupta SK, Karim K (2003) Continuous biotransformation and removal of nitrophenols under denitrifying conditions. Water Res 37:2953–2959
Gupta VK, Sharma S, Yadav IS, Mohan D (1998) Utilization of bagasse fly ash generated in the sugar industry for the removal and recovery of phenol and p-nitrophenol from wastewater. J Chem Technol Biotechnol 71:180–186
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Ali I, Saini VK (2004) Removal of chlorophenols from wastewater using red mud: an aluminum industry waste. Environ Sci Technol 38:4012–4018
Inada M, Tsujimoto H, Eguchi Y, Enomoto N, Hojo J (2005) Microwave-assisted zeolite syntheses from coal fly ash in hydrothermal process. Fuel 84:1482–1486
Joint committee on powder diffraction standards, JCPDS (1971), Index (Inorganic to the powder diffraction file), Publication PDIS-211, Newton square, PA
Juang RS, Lin SH (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manage 90:1336–1349
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Lin C-F, His H-C (1995) Resource recovery of waste fly ash: synthesis of zeolite-like materials. Environ Sci Technol 29:1109–1117
Mall ID, Srivastava VC, Swamy MM, Prasad B, Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetic and thermodynamics. Colloid Surf 272:89–104
Ozcan Safa A, Erdem B, Ozcan A (2005) Adsorption of acid blue 193 from aqueous solutions onto BTMA-bentonite. Colloid Surf A 266:73–81
Pan B, Du W, Zhang W, Zhang X, Zhang Q, Pan B, Lv L, Zhange Qu, Chen J (2007) Improved adsorption of 4-nitrophenol onto a novel hyper-cross-linked polymer. Environ Sci Technol 41:5057–5062
Pandit AB, Gogate PR (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597
Release and pollution prevention report (2000) US Environmental Protection Agency; Washington, DC
Sarkar M, Acharya PK, Bhattacharya B (2003) Modelling the adsorption kinetics of some priority organic pollutants in water from diffusion and activated energy parameters. J Colloid Interface Sci 266:28–32
Sarkar M, Acharya P, Bhattacharya B (2005) Removal characteristics of some priority organic pollutants from water in a fixed bed fly ash column. J Chem Technol Biotechnol 80:1349–1355
Sathishkumar M, Binupriya AR, Kavitha D, Selvakumar R, Sheema KK, Choi JG, Yun SE (2008) Organic micro-pollutant removal in liquid-phase using carbonized silk cotton hull. J Environ Sci 20(9):1046–1054
Schwarz JA, Noh JS (1990) Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 28:675–682
Shah BA, Shah AV, Singh RR, Patel NB (2009) Sorptive removal of nickel onto weathered basaltic andesite products: kinetics and isotherms. J Environ Sci Health Part A 44:880–895
Singh BK, Nayak PS (2004) Sorption equilibrium studies of toxic nitro-substituted phenols on fly ash. Adsorpt Sci Technol 22:295–310
Toxic Release Inventory (1992) Toxicological profile for nitrophenols: 2-nitrophenols and 4-nitrophenols. Office of toxic substances, Washington
Treacy MMJ, Higgins JB (2001) Collection of simulated XRD powder patterns for zeolites. Fourth Revised Edition Published on behalf of the Structure Commission of the ‘International Zeolite Association’, Elsevier, Amsterdam, London, New York, Oxford, Paris, Shannon, Tokyo
Tutem E, Apak R, Unal CF (1998) Adsorptive removal of chlorophenols from water by bituminous shale. Water Res 32:2315–2324
Vucinic D, Miljanovic I, Rosic A, Lazic P (2003) Effect of Na2O/SiO2 mole ratio on the crystal type of zeolite synthesized from coal fly ash. J Serb Chem Soc 68:471–478
Wang S, Wu H (2006) Review environmental-benign utilisation of fly ash as low-cost adsorbents. J Hazard Mater B 136:482–501
Wang Y, Guo Y, Yang Z, Cai H, Xavier Q (2003) Synthesis of zeolites using fly ash and their application in removing heavy metals from water. Sci China Ser: D 46:967–976
Wang S, Soudi M, Li L, Zhu ZH (2006) Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater. J Hazard Mater B 133:243–251
Wang S, Li L, Zhu ZH (2007) Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J Hazard Mater B 139(2):254–259
Weber WJJr, Asce AM, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Di V Am Soc Ci V Eng 89:31–59
Zheng Z, Tang D, Lin K, Luan J, Zhang J (2007) Adsorption of p-nitrophenol from aqueous solutions onto activated carbon fiber. J Hazard Mater 143:49–56
Zümriye A (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Acknowledgments
The authors are grateful to the Special Assistance Programme meritorious fellowship, UGC, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsbile editor: Euripides Stephanou
Rights and permissions
About this article
Cite this article
Shah, B., Tailor, R. & Shah, A. Zeolitic bagasse fly ash as a low-cost sorbent for the sequestration of p-nitrophenol: equilibrium, kinetics, and column studies. Environ Sci Pollut Res 19, 1171–1186 (2012). https://doi.org/10.1007/s11356-011-0638-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0638-6



