Abstract
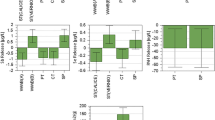
The present study enlightens the impounding of ortho-chlorophenol (OCP) onto zeolitic composites derived from agricultural waste Bagasse Fly Ash (BFA). The OCP impounding was enhanced by modifying native BFA by electrolyte supported microwave hydrothermal treatment (MZBFA) and magnetic modification (MMZBFA). The synthesized sorbents were characterized by instrumental techniques (XRF, PXRD, SEM and FTIR). The adsorption process was optimized under four different process variables like: pH (4–7), agitation time (30–120 min), initial sorbate concentration (\({50-150\,{\rm mg}\,{\rm L}^{-1}}\)), sorbent dosage (\({1-4\,{\rm g}\,{\rm L}^{-1}}\)) based on Box-Behnken design with response surface methodology. The highest predicted adsorption capacities at pH 7 with \({1\,{\rm g}\,{\rm L}^{-1}}\) sorbent dose for MZBFA and MMZBFA were found to be 29.95 and \({31.94\,{\rm mg}\,{\rm g}^{-1}}\) for agitation time of 75 and 120 min and initial sorbate concentration 150 and \({100\,{\rm mg}\,{\rm g}^{-1}}\) , respectively, which were approximated with laboratory results. The isotherm data and kinetic data were best described by Langmuir model and pseudo-second-order model, respectively. The sorbents are stable after three cyclic runs when regenerated with 0.5M NaOH solution.
Similar content being viewed by others
References
Hank D., Azi Z., Ait Hocine S., Chaalal O., Hellal A.: Optimization of phenol adsorption onto bentonite by factorial design Methodology. J. Ind. Eng. Chem. 20, 2256–2263 (2014)
Lin S.-H., Juang R.-S.: Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 90, 1336–1349 (2009)
Yang J., Zhou M., Zhao Y., Zhang C., Hu Y.: Electrosorption driven by microbial fuel cells to remove phenol without external power supply. Bioresour. Technol. 150, 271–277 (2013)
Soto M.L., Moure A., Domínguez H., Parajó J.C.: Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 105, 1–27 (2011)
El-Naas M.H., Al-Zuhair S., Alhaija M.A.: Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem. Eng. J. 162, 997–1005 (2010)
Shah B.A., Shah A.V., Tailor R.: Adaptation of bagasse fly ash a sugar industry solid waste into zeolitic material for the uptake of phenol. Environ. Prog. Sustain 30(3), 359–367 (2011)
Huang J., Wanga X., Jina Q., Liua Y., Wang Y.: Removal of phenol from aqueous solution by adsorption onto OTMAC-modified attapulgite. J. Environ. Manag. 84, 229–236 (2007)
Ku Y., Lee K.C., Wang W.: Removal of Phenols from Aqueous solutions by Purolite A-510 Resin. Sep. Sci. Technol. 39, 911–923 (2004)
Tak B., Tak B., Kim Y., Park Y., Yoon Y., Min G.: Optimization of color and COD removal from livestock wastewater by electrocoagulation process: Application of Box-Behnken design (BBD). J. Ind. Eng. Chem. 28, 307–315 (2015)
Kamcharoen A., Champreda V., Eurwilaichitr L., Boonsawang P.: Screening and optimization of parameters affecting fungal pretreatment of oil palm empty fruit bunch (EFB) by experimental design. Int. J. Energy Environ. Eng. 5, 303–312 (2014)
Oliveira L.C., Petkowicz D.I., Smaniotto A., Pergher S.: Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Res. 38(17), 3699–3704 (2004)
Noh J.S., Schwarz J.A.: Estimation of the point of zero charge of simple oxides by mass titration. J. Colloid Interface Sci. 130, 157–164 (1989)
Yue L., Wang L., Shi F., Guo J., Yang J., Lian J., Luo X.: Application of response surface methodology to the decolorization by the electrochemical process using \({{\rm FePMo}_{12}{\rm O}_{40}}\) catalyst. J. Ind. Eng. Chem. 21, 971–979 (2015)
Thirugnanasambandham K., Sivakumar V., Maran J. P., Kandasamy S.: Chitosan based grey wastewater treatment—A statistical design Approach. Carbohydr. Polym. 99, 593–600 (2014)
Thirugnanasambandham K., Sivakumar V.: An eco-friendly approach for copper (II) ion adsorption onto cotton seed cake and its characterization: Simulation and validation. J. Taiwan Inst. Chem. Eng. 50, 198–204 (2015)
Vogel A.I.: A Text Book of Quantitative Analysis, 5th edn. ELBS, London (1989)
Brasquet C.F., Kadirvelu K., Le C.P.: Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir 16, 8404–8409 (2000)
Huo H., Lin H., Dong Y., Cheng~Wang H.H., Cao L.: Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. J. Hazard. Mater. 229–230, 292–297 (2012)
Joint committee on powder diffraction standards, Index (Inorganic to the powder diffraction file, Publication PDIS-211; Newton Square, PA (1971)
Nah I.W.K., Hwang Y., Shul Y.G.: A simple synthesis of magnetically modified zeolite. Powder Technol. 177(2), 99–101 (2007)
Shah B.A., Shah A.V., Mistry C.B., Tailor R.V., Patel H.D.: Surface Modified Bagasse Fly Ash Zeolites for Removal of Reactive Black-5. J. Dispers. Sci. Technol. 32(9), 1247–1255 (2011)
Inada M., Tsujimoto H., Eguchi , Enomoto N., Hojo J.: Microwave assisted zeolite synthesis from coal fly ash in hydrothermal process. Fuel 84, 482–1486 (2005)
Sapawe N., Jalil A.A., Triwahyono S., Shah M.I.A., Jusoh R., Salleh N.F.M., Hameed B.H., Karim A.H.: Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue. Chem. Eng. J. 229, 388–398 (2013)
Shah B.A., Mistry C.B., Shah A.V.: Sequestration of Cu(II) and Ni(II) from wastewater by synthesized zeolitic materials: Equilibrium, kinetics and column dynamics. Chem. Eng. J. 220, 172–184 (2013)
Sari A., Tuzen M., Soylak M.: Adsorption of Pb(II) and Cr(III) from aqueous solution on Celtek clay. J. Hazard. Mater. 144, 41–46 (2007)
Anirudhan T.S., Shainy F.: Adsorption behaviour of 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nano cellulose composite for cadmium(II) from aqueous solutions. J. Ind. Eng. Chem. 32, 157–166 (2015)
Shah B., Tailor R., Shah A.: Sorptive sequestration of 2-chlorophenol by zeolitic materials derived from bagasse fly ash. J. Chem. Technol. Biotechnol. 86, 1265–1275 (2011)
Kao P.N., Tzeng J.H., Huang T.L.: Removal of chlorophenols from aqueous solution by fly ash. J. Hazard. Mater. 76, 237–249 (2000)
Coughlin R.W., Ezra F.S.: Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 2, 291–297 (1968)
Hunter C.A., Sanders J.K.M.: The nature of \({{\pi}}\) –\({{\pi}}\) interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990)
Liu Q.-S., Zheng T., Wang P., Jiang J.-P., Li N.: Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 157, 348–356 (2010)
Ghaedi, M. Shojaeipour.; Ghaedi, E.A.M.; Sahraei, Reza.: Isotherm and kinetics study of malachite green adsorption onto copper nanowires loaded on activated carbon: Artificial neural network modeling and genetic algorithm optimization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 142, 135–149 (2015) 900
Khare P., Kumar A.: Removal of phenol from aqueous solution using carbonized Terminalia chebula-activated carbon: process parametric optimization using conventional method and Taguchi’s experimental design, adsorption kinetic, equilibrium and thermodynamic study. Appl. Water Sci. 2, 317–326 (2012)
Tak B.-y., Tak B.-s., Kim Y.-j., Park Y.-j., Yoon Y.-h., Min G.-h.: Optimization of color and COD removal from livestock wastewater by electrocoagulation process: Application of Box-Behnken design (BBD). J. Ind. Eng. Chem. 28, 307–315 (2015)
Langmuir I.: The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 38, 2221–2295 (1916)
Ghasemi M., Khosroshahy M.Z., Abbasabadi A.B., Ghasemi N., Javadian H., Fattahi M.: Microwave-assisted functionalization of Rosa Canina-L fruits activated carbon with tetraethylenepentamine and its adsorption behaviour toward Ni(II) in aqueous solution: Kinetic, equilibrium and thermodynamic studies. Powder Technol. 274, 362–371 (2015)
Zheng L., Wang C., Shu Y., Yan X., Li L.: Utilization of diatomite/chitosan–Fe (III) composite for the removal of anionic azo dyes from wastewater: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 468, 129–139 (2015)
Hameed B.H., Rahman A.A.: Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 160, 576–581 (2008)
Vijayaraghavan K., Padmesh T.V., Palanivelu K., Velan M.: Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three parameter isotherm models. J. Hazard. Mater. 133, 304–308 (2006)
Amirnia S., Ray M.B., Margaritis A.: Copper ion removal by Acer saccharum leaves in a regenerable continuous-flow column. Chem. Eng. J. 287, 755–764 (2016)
Ghaedi M., Rozkhoosh Z., Asfaram A., Mirtamizdoust B., Mahmoudi Z., Bazrafshan A.A.: Comparative studies on removal of Erythrosine using ZnS and AgOH nanoparticles loaded on activated carbon as adsorbents: Kinetic and isotherm studies of adsorption. Spectrochim. Acta A Mol. Biomol. Spectrosc. 138, 176–186 (2015)
Weber T.W., Chakraborti R.K.: Pore and solid diffusion models for fixed bed adsorbers. Am. Inst. Chem. Eng. J. 20, 228–238 (1974)
Gupta V.K., Ali I., Saini V.K.: Removal of chlorophenols from wastewater using red mud: an aluminum industry waste. Environ. Sci. Technol. 38, 4012–4018 (2004)
Shiundu P.M., Mbui D.N., Ndonye R.M., Kamau G.M.: Adsorption and detection of some phenolic compounds by rice husk ash of Kenyan origin. J. Environ. Monit. 4, 978–984 (2002)
Javadian H., Vahedian P., Toosi M.: Adsorption characteristics of Ni(II) from aqueous solution and industrial wastewater onto polyaniline/HMS nanocomposite powder. Appl. Surf. Sci. 284, 13–22 (2013)
Elmoubarki R., Mahjoubi F.Z., Tounsadi H., Moustadraf J., Abdennouri M., Zouhri A., ElAlbani A., Barka N.: Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour. Ind. 9, 16–29 (2015)
Temkin M.J., Pyzhev V.: Recent modifications to Langmuir Isotherms. Acta Physiochim. USSR 12, 217–222 (1940)
Blazquez G., Martin-Lara M.A., Tenorio G., Calero M.: Batch biosorption of lead (II) from aqueous solutions by olive tree pruning waste: Equilibrium, kinetics and thermodynamic study. Chem. Eng. 168, 170–177 (2011)
Lagergren S.: About the theory of the so-called adsorption of soluble substances. Kun. Sven. Vetenskapsakad. Handl. 24, 1–39 (1898)
Belaid K.D., Kacha S., Kameche M., Derriche Z.: Adsorption kinetics of some textile dyes onto granular activated carbon. J. Environ. Chem. Eng. 1, 496–503 (2013)
Ho Y.S., Mckay G.: Pseudo-second-order model for sorption processes. Process Biochem. 34, 451–465 (1999)
Al Hamouz O.C.S.: Synthesis and Characterization of a Novel Series of Cross-Linked (Phenol, Formaldehyde, Alkyldiamine) Terpolymers for the Removal of Toxic Metal Ions from Wastewater. Arab. J. Sci. Eng. 41, 119–133 (2015)
Barka N., Abdennouri M., El Makhfouk M., Qourzal S.: Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 1, 144–149 (2013)
Chien S.H., Clayton W.R.: Application of Elovich equation to the kinetics of phosphate release and sorption on soil. Soil Sci. Soc. Am. 44, 265–268 (1980)
Amirnia S., Ray M.B., Margaritis A.: Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor–biosorption system. Chem. Eng. J. 264, 863–872 (2015)
Shu J., Wang Z., Huang , Ni~Huang Y., Ren C., Zhang W.: Adsorption removal of Congo red from aqueous solution by polyhedral \({{\rm Cu}_{2}{\rm O}}\) nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloys Compd. 633, 338–346 (2015)
Shah B.A., Shah A.V., Singh R.R., Patel N.B.: Sorptive removal of nickel onto weathered basaltic andesite products: kinetics and isotherms. J. Environ. Sci. Health A 44, 880–895 (2009)
Ncibi M.C., Gaspard S., Sillanpää M.: As-synthesized multi-walled carbon nanotubes for the removal of ionic and non-ionic surfactants. J. Hazard. Mater. 286, 195–203 (2015)
Anirudhan T.S., Sreekumari S.S., Brigle C.D.: Removal of phenols from water and petroleum industry refinery effluents by activated carbon obtained from coconut coir pith. Adsorption 15, 439–451 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, B.A., Pandya, D.D. & Shah, H.A. Impounding of ortho-Chlorophenol by Zeolitic Materials Adapted from Bagasse Fly Ash: Four Factor Three Level Box-Behnken Design Modelling and Optimization. Arab J Sci Eng 42, 241–260 (2017). https://doi.org/10.1007/s13369-016-2294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-016-2294-0