Abstract
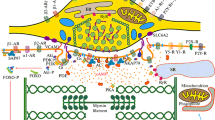
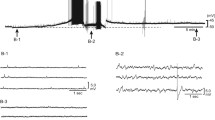
The discharge of vasoconstrictor pathways arising in the CNS is largely unmodified as it passes through the sympathetic ganglia to the vasculature. The underlying synaptic events have been revealed by intracellular recordings from sympathetic paravertebral ganglion cells in the course of ongoing and reflex activity in anesthetized animals, first made in Skok’s Laboratory in Kyiv (Ukraine). Each preganglionic neuron diverges to contact a number of post-ganglionic neurons, on each of which several pre-ganglionic inputs converge. However, only suprathreshold “strong,” or “dominant” synapses are effective in transmitting the CNS signals. Strong synapses differ from the other subthreshold “weak,” or “accessory” inputs: (a) excitatory synaptic currents are >1 nA in their amplitude, (b) 3 to ≈>30 times more quanta of acetylcholine are released, (c) pre-synaptic Ca2+ entry through channels resistant to all-known antagonists triggers acetylcholine release, and (d) post-synaptic Ca2+ entry boosts and prolongs the nicotinic current. While the majority of postganglionic neurons have only one strong input, a proportion receives two or, rarely, three such inputs. In cells with multiple strong inputs, an equivalent number of discrete Ca2+ currents can be evoked at distinct foci electrically distant from the soma, suggesting that each strong input has a unique dendritic association with a cluster of Ca2+ channels. When strong preganglionic inputs are destroyed, residual weak synapses sprout and rapidly restore the suprathreshold connections. While much remains to be discovered about how strong synapses are established, their high safety factor ensures the wide and secure distribution of vasoconstrictor command signals from the CNS.
Similar content being viewed by others
References
J. H. Coote, “Landmarks in understanding the central nervous control of the cardiovascular system,” Exp. Physiol., 92, 3–18 (2007).
P. G. Guyenet, “The sympathetic control of blood pressure,” Nat. Rev. Neurosci., 7, 335–346 (2006).
K. Dembowsky, J. Czachurski, and H. Seller, “An intracellular study of the synaptic input to sympathetic preganglionic neurons of the third thoracic segment of the cat,” J. Auton. Nerv. Syst., 13, 201–244 (1985).
E. M. McLachlan and G. D. S. Hirst, “Some properties of preganglionic neurons in the upper thoracic spinal cord of the cat,” J. Neurophysiol., 43, 1251–1265 (1980).
P. Sah and E. M. McLachlan, “Membrane properties and synaptic potentials in rat sympathetic preganglionic neurons studied in horizontal spinal cord slices in vitro,” J. Auton. Nerv. Syst., 53, 1–15 (1995).
W. Jänig, The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis, Cambridge Univ. Press, New York (2006).
R. A. Dampney, M. J. Coleman, M. A. Fontes, et al., “Central mechanisms underlying short-and long-term regulation of the cardiovascular system,” Clin. Exp. Pharmacol. Physiol., 29, 261–268 (2002).
D. W. Wheeler, P. H. Kullmann, and J. P. Horn, “Estimating use-dependent synaptic gain in autonomic ganglia by computational simulation and dynamic-clamp analysis,” J. Neurophysiol., 92, 2659–2671 (2004).
J. N. Langley, “The autonomic nervous system,” Brain, 26, 1–26 (1903).
W. Feldberg and J. H. Gaddum, “The chemical transmitter at synapses in a sympathetic ganglion,” J. Physiol., 81, 305–319 (1934).
V. I. Skok, “Channel-blocking mechanism ensures specific blockade of synaptic transmission,” Neuroscience, 17, 1–9 (1986).
V. I. Skok, “Nicotinic acetylcholine receptors in autonomic ganglia,” Auton. Neurosci., 97, 1–11 (2002).
R. J. Evans, V. Derkach, and A. Surprenant, “ATP mediates fast synaptic transmission in mammalian neurons,” Nature, 357, 503–505 (1992).
H. Inokuchi and E. M. McLachlan, “Lack of evidence for P2X-purinoceptor involvement in fast synaptic responses in intact sympathetic ganglia isolated from guinea-pigs,” Neuroscience, 69, 651–659 (1995).
V. I. Skok, Physiology of Autonomic Ganglia, Igaku Shoin, Tokyo (1973).
V. I. Skok and A. I. Ivanov, “What is the ongoing activity of sympathetic neurons?” J. Auton. Nerv. Syst., 7, 263–270 (1983).
A. Ivanov and D. Purves, “Ongoing electrical activity of superior cervical ganglion cells in mammals of different sizes,” J. Comp. Neurol., 284, 398–404 (1989).
E. M. McLachlan, P. J. Davies, H.-J. Häbler, et al., “On-going and reflex synaptic events in rat superior cervical ganglion cells,” J. Physiol., 501, 165–182 (1997).
E. M. McLachlan, H.-J. Häbler, J. Jamieson, et al., “Analysis of the periodicity of synaptic events in neurons in the superior cervical ganglion of anaesthetized rats,” J. Physiol., 511, 461–478 (1998).
V. G. Macefield and B. G. Wallin, “Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurons to human skin,” J. Physiol., 516, 303–314 (1999).
M. E. Holman and G. D. S. Hirst, “Junctional transmission in smooth muscle and the autonomic nervous system,” in: Handbook of Physiology, Sect. 1, The Nervous System, E. R. Kandel, (ed.), Am. Physiol. Society, Bethesda (1977), pp. 417–462.
J. Jamieson, H. D. Boyd, and E. M. McLachlan, “Simulations to derive membrane resistivity in three phenotypes of guinea pig sympathetic neuron,” J. Neurophysiol., 89, 2430–2440 (2003).
D. Purves, “Functional and structural changes in mammalian sympathetic neurones following interruption of their axons,” J. Physiol., 252, 429–463 (1975).
A. S. Finkel and S. Redman, “Theory and operation of a single microelectrode voltage clamp,” J. Neurosci. Meth., 11, 101–127 (1984).
J. G. Blackman and R. D. Purves, “Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig,” J. Physiol., 203, 173–198 (1969).
G. D. S. Hirst and E. M. McLachlan, “Post-natal development of ganglia in the lower lumbar sympathetic chain of the rat,” J. Physiol., 349, 119–134 (1984).
P. Jobling and I. L. Gibbins, “Electrophysiological and morphological diversity of mouse sympathetic neurons,” J. Neurophysiol., 82, 2747–2764 (1999).
D. Purves, E. Rubin, W. D. Snider, et al., “Relation of animal size to convergence, divergence, and neuronal number in peripheral sympathetic pathways,” J. Neurosci., 6, 158–163 (1986).
W. D. Snider, “Rostrocaudal differences in dendritic growth and synaptogenesis in rat sympathetic chain ganglia,” J. Comp. Neurol., 244, 245–253 (1986).
D. Purves and R. I. Hume, “The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells,” J. Neurosci., 1, 441–452 (1981).
I. L. Gibbins, P. Jobling, J. P. Messenger, et al., “Neuronal morphology and the synaptic organisation of sympathetic ganglia,” J. Auton. Nerv. Syst., 81, 104–109 (2000).
E. M. McLachlan, “An analysis of the release of acetylcholine from preganglionic nerve terminals,” J. Physiol., 245, 447–466 (1975).
E. M. McLachlan, “The statistics of transmitter release at chemical synapses,” in: International Review of Physiology-Neurophysiology III, R. Porter (ed.), Univ. Park Press, Baltimore (1978), pp. 49–117.
D. R. Ireland, P. J. Davies, and E. M. McLachlan, “Calcium channel subtypes differ at two types of cholinergic synapse in lumbar sympathetic neurons of guinea-pigs,” J. Physiol., 514, 59–69 (1999).
P. Jobling, I. L. Gibbins, R. J. Lewis, et al., “Differential expression of calcium channels in sympathetic and parasympathetic preganglionic inputs to neurons in paracervical ganglia of guinea-pigs,” Neuroscience, 127, 455–466 (2004).
G. R. Seabrook and D. J. Adams, “Inhibition of neurally evoked transmitter release by calcium channel antagonists in rat parasympathetic ganglia,” Br. J. Pharmacol., 97, 1125–1136 (1989).
A. B. Smith and T. C. Cunnane, “Calcium channels controlling acetylcholine release in the guinea-pig isolated anterior pelvic ganglion: an electrophysiological study,” Neuroscience, 94, 891–896 (1999).
A. B. Smith, L. Motin, N. A. Lavidis, et al., “Calcium channels controlling acetylcholine release from preganglionic nerve terminals in rat autonomic ganglia,” Neuroscience, 95, 1121–1127 (2000).
S. Mochida, H. Saisu, H. Kobayashi, et al., “Impairment of syntaxin by botulinum neurotoxin C1 or antibodies inhibits acetylcholine release but not Ca2+ channel activity,” Neuroscience, 65, 905–915 (1995).
P. M. Lundy and R. Frew, “Effect of omega-agatoxin-IVA on autonomic neurotransmission,” Eur. J. Pharmacol., 261, 79–84 (1994).
J. F. Cassell and E. M. McLachlan, “The effect of a transient outward current (IA) on synaptic potentials in sympathetic ganglion cells of the guinea-pig,” J. Physiol., 374, 273–288 (1986).
V. A. Derkach, A. A. Selyanko, and V. I. Skok, “Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurons,” J. Physiol., 336, 511–526 (1983).
V. A. Derkach, R. A. North, A. A. Selyanko, et al., “Single channels activated by acetylcholine in rat superior cervical ganglion,” J. Physiol., 388, 141–151 (1987).
G. D. S. Hirst and E. M. McLachlan, “Development of dendritic calcium currents in ganglion cells of the rat lower lumbar sympathetic chain,” J. Physiol., 377, 349–368 (1986).
R. J. Callister, J. R. Keast, and P. Sah, “Ca2+-activated K+ channels in rat otic ganglion cells: role of Ca2+ entry via Ca2+ channels and nicotinic receptors,” J. Physiol., 500, 571–582 (1997).
H. J. Häbler, W. Jänig, and M. Michaelis, “Respiratory modulation in the activity of sympathetic neurones,” Prog. Neurobiol., 43, 567–606 (1994).
E. M. McLachlan, “Transmission of signals through sympathetic ganglia-modulation, integration or simply distribution?” Acta Physiol. Scand., 177, 227–235 (2003).
H. J. Habler, E. M. McLachlan, J. Jamieson, et al., “Synaptic responses evoked by lower urinary tract stimulation in superior cervical ganglion cells in the rat,” J. Urol., 161, 1666–1671 (1999).
K. Liestol, J. Maehlen, and A. Nja, “Two types of synaptic selectivity and their interrelation during sprouting in the guinea-pig superior cervical ganglion,” J. Physiol., 384, 233–245 (1987).
J. G. Murray and J. W. Thompson, “The occurrence and function of collateral sprouting in the sympathetic nervous system of the cat,” J. Physiol., 135, 133–162 (1957).
J. W. Lichtman and D. Purves, “The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early postnatal life,” J. Physiol., 301, 213–228 (1980).
I. L. Gibbins, P. Jobling, E. H. Teo, et al., “Heterogeneous expression of SNAP-25 and synaptic vesicle proteins by central and peripheral inputs to sympathetic neurons,” J. Comp. Neurol., 459, 25–43 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Neirofiziologiya/Neurophysiology, Vol. 39, Nos. 4/5, pp. 294–301, July–October, 2007.
Rights and permissions
About this article
Cite this article
McLachlan, E.M. Synaptic transmission in sympathetic vasoconstrictor pathways and its modification after injuries. Neurophysiology 39, 251–258 (2007). https://doi.org/10.1007/s11062-007-0035-4
Issue Date:
DOI: https://doi.org/10.1007/s11062-007-0035-4