Abstract
The sympathetic preganglionic neurons (SPNs) play a key role in the sympathetic nervous system. Previous reports have suggested that norepinephrine (NE) directly affects SPNs via both inhibitory hyperpolarization interactions mediated by α2 receptors and excitatory depolarization interactions mediated by α1 receptors. It remains poorly understood, however, whether the excitability of SPNs can be inhibited indirectly (presynaptically) as well as directly (postsynaptically). We intracellularly recorded 41 SPNs using the whole-cell patch-clamp technique in spinal cord slice preparations of neonatal rats. We examined the effects of NE or dexmedetomidine hydrochloride (Dxm) (α2-adrenergic receptor agonist) on SPNs by analyzing the excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs). EPSPs were dominant in 15 SPNs (EPSP-SPNs) and IPSPs were dominant in 7 SPNs (IPSP-SPNs) at baseline. We were unable to analyze the postsynaptic potentials in the other 19 SPNs, due to high frequency of action potential firings (firing-SPNs). At baseline, the membrane potentials and resistances of each type of SPN were similar. NE (1 μM) gradually depolarized the EPSP-SPNs and IPSP-SPNs (P < 0.001) and NE significantly increased the EPSP frequency of the EPSP-SPNs (P < 0.05). Dxm (10 nM) after application of NE decreased the EPSP frequency of the EPSP-SPNs (P < 0.001) and the EPSP voltage and IPSP voltage of the IPSP-SPNs (P < 0.05). In 5 of the 19 firing-SPNs, NE induced membrane hyperpolarization (P < 0.05) and completely inhibited firings. Dxm had no effect in these neurons. The SPNs received inhibitory modulation through α2-adrenergic receptors. Some SPNs can be directly inhibited via effects independent of the α2 receptors.
Similar content being viewed by others
Introduction
Sympathetic hyperactivity is a risk factor for cardiovascular disease. Elevated plasma norepinephrine (NE) levels are reported to be associated with increased mortality in patients and animal models [1, 2]. The effects of the sympathetic preganglionic neurons (SPNs) at the final points to each sympathetic organ in the sympathetic system play a key role in preventing sympathetic hyperactivity. Alpha2 (α2)-adrenergic receptors are highly concentrated in the SPNs in the intermediolateral cell column (IML) of the thoracic spinal cord and NE-containing axons innervated to the IML regions. [3,4,5]. Previous reports suggested that NE directly (postsynaptically) inhibits SPNs through interactions mediated by α2-adrenergic receptors.
NE applied to the SPNs of rat spinal cord micro-iontophoretically brought about hyperpolarization [6]. When α2-adrenergic, α1-adrenergic and β-receptor antagonists were superfused after NE, only the α2-adrenergic antagonists blocked the inhibitory effects on the SPNs. The effects reported in other studies have been similar [7, 8]. Nishi et al. [9] found that noradrenergic neurons can affect SPNs via both inhibitory hyperpolarization interactions mediated by α2 receptors and excitatory depolarization interactions mediated by α1 receptors. It remains unclear, however, whether SPNs are also inhibited indirectly (presynaptically) by synaptic transmission.
In our preliminary study, the SPNs showed spontaneous excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs) during whole-cell patch-clamp recordings (unpublished data). Electrophysiologically, the indirect excitatory effects are transmitted by EPSP and the inhibitory effects are transmitted by IPSP. There are few data, however, clearly showing the direct and indirect (presynaptic) effects of NE or the associations between the excitatory/inhibitory effects of NE and dexmedetomidine hydrochloride (Dxm) on SPNs and α2-adrenergic receptors. In this study we sought to clarify the inhibitory action of α2-adrenergic receptors agonist/antagonist on SPNs by examining the direct and indirect responses of SPNs to NE and Dxm.
Methods
Intracellular recordings
Wistar rats of either sex (0–6 days old) were used for the experiments. The spinal cord was excised from each rat after including anesthesia with ether. Transverse sections from segments Th1 to Th2 were resected with scissors and placed in artificial cerebrospinal fluid (ACSF) (25 °C). The methods used to isolate and perfuse the SPNs of the excised spinal cord have been described in detail in a previous report [10]. All experimental protocols were approved by the institutional review board of our facility. Intracellular recordings of SPNs were taken by the whole-cell patch-clamp technique. SPNs were identified by their all-or-none antidromic responses to mainly Th2 ventral root stimulation applied with a suction electrode (1–10 V, duration 100 μs). The methods used to recording the SPNs have also been described in detail previously [10]. The EPSPs and IPSPs in the intracellular recordings of the SPNs were identified as small depolarizing and hyperpolarizing changes in membrane potential with steep starts, as mentioned before [11, 12]. An EPSP was defined as a depolarizing potential change with a fast-rising phase of more than 0.4 v/s. The amplitudes of the EPSPs were determined by voltage differences of more than 0.4 mV between the rising points and peaks. Recording noise was defined as a depolarization below 0.3 mV. Similarly, an IPSP was defined as a hyperpolarizing potential change with a fast-rising phase of more than 0.2 v/s. The amplitude of the IPSPs were determined by the voltage differences of more than 0.4 mV between the descending points and peaks. Recording noise was defined as a depolarization below 0.3 mV.
Drugs and protocols
All of the agents dissolved in standard ACSF. The agents and solutions were applied to the spinal cord slice preparation by superfusion at a rate of 3 ml/min. The NE (Daiichi Sankyo Co., Tokyo, Japan) and Dxm (Hospira Japan Co., Ltd., Osaka, Japan) concentrations were selected based on data from previous studies [13, 14]. The SPNs were superfused with 1 μM NE for 5 min. To test the contribution of the α2 receptor, SPNs were superfused with 10 nM Dxm after washout of NE for 10 min by a similar method. To distinguish the direct postsynaptic effects of NE on the SPNs, superfusion with 0.5 μM tetrodotoxin (TTX), block action potential-dependent transmitter release, was commenced 10 min before the start of the NE superfusion. After this pretreatment with TTX, the SPNs were superfused with 1 μM NE for 5 min in the presence of TTX.
Statistical analysis
All data were expressed as means ± SD. The statistical significance differences (P < 0.05) were analyzed by repeated-measure analysis of variance, Tukey's honestly significant difference.
Results
SPNs spontaneously presented action potentials (APs) and postsynaptic potentials such as EPSPs and IPSPs. In previous reports, we classified SPNs into two subtypes: (1) silent neurons exhibiting no spontaneous APs and (2) firing neurons exhibiting spontaneous APs [10]. To elucidate the characteristics of the postsynaptic potentials of the SPNs, we analyzed the frequency and amplitude of EPSPs and IPSPs. When working with SPNs with high-frequency APs (more than 0.5 Hz in some of the firing neurons), we had few available means to clarify the characteristics of the EPSPs and IPSPs. After the establishment of whole-cell patch-clamp recordings, some of the SPNs predominantly showed EPSPs (EPSP-SPNs) (EPSP/IPSP > 1) at baseline and some of the SPNs predominantly showed IPSPs (IPSP-SPNs) (EPSP/IPSP < 1). We recorded a total of 41 SPNs: 15 EPSP-SPNs, 7 IPSP-SPNs and 19 firing neurons with high-frequency firing. The membrane potentials of the three SPN types were similar (−46.6 ± 3.0 mV in EPSP-SPNs, –45.0 ± 1.1 mV in IPSP-SPNs −44.5 ± 3.8 mv in firing neurons). No significant difference was found in the input resistances among the three SPN types (246.1 ± 200.1 MΩ in EPSP-SPNs, 167.3 ± 87.3 MΩ in IPSP-SPNs and 284.8 ± 164.7 MΩ in firing-SPNs). The frequencies of the EPSPs and IPSPs were 0.76 ± 0.35 Hz and 0.27 ± 0.21 Hz in the EPSP-SPNs and 0.85 ± 0.90 Hz and 1.05 ± 1.12 Hz in the IPSP-SPNs. The firing rate of the firing-SPNs was 2.08 ± 1.15 Hz. All data at control in Table 1 show stable-phase data after whole-cell patch-clamp.
Effects of noradrenaline and dexmedetomidine hydrochloride on EPSP-SPNs
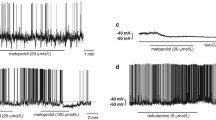
An examination of the effects of NE on the EPSP-SPNs revealed a gradual significant depolarization of the EPSP-SPNs together with the appearance of spontaneous action potentials from 3 to 4 min after the start of the 1 μM NE superfusion (Fig. 1a, Table 1). The input resistances of the EPSP-SPNs increased, but not to a significant degree. The EPSPs significantly increased during the NE superfusion (Table 1). The amplitudes of the EPSPs and IPSPs showed no significant change after the NE superfusion (Table 1). At 5 min after the NE washout, the membrane potential recovered to −43.5 ± 4.2 mV (Fig. 1a, b-1, b-2). All data at washout show stable-phase data after washout of NE.
Responses of EPSP-SPNs to NE and Dxm. a EPSP-SPNs were gradually depolarized from 3 to 5 min after the commencement of the superfusion with 1 μM NE. The responses recovered in about 10 min after washout. Dxm brought about no change of the membrane potential. To compare input resistances and EPSPs at the same membrane potential, the membrane potential of the neuron was adjusted to the same level as that of control by negative current injection at b-2. b-1 EPSPs in a faster sweep representation at control. b-2 Increase of EPSPs by NE. The application of NE increased the frequency and augmented the voltage of the EPSPs. b-3 Attenuation of EPSPs by Dxm. Dxm decreased both the frequency and voltage of the EPSPs
We next examined the effects of Dxm on the 9 EPSP-SPNs after the NE superfusion in order to clarify the noradrenergic effects exerted upon the EPSP-SPNs via α2 receptor. Dxm elicited no significant change of the EPSP-SPN membrane potential compared with that after the NE washout (−44.0 ± 4.1 mV vs. −43.5 ± 4.2 mV). Dxm did, however, significantly decrease the number of EPSPs. The amplitudes of the EPSPs and IPSPs after NE did not significantly differ from those after Dxm (Fig. 1a, b-3) (Table 1). All data after Dxm in Table 1 show peak-phase data after Dxm.
Effects of noradrenaline and dexmedetomidine hydrochloride on IPSP-SPNs
Next, in a similar examination of the effects of NE on IPSP-SPNs, a significant depolarization of the IPSP-SPNs appeared from 3 to 4 min after the start of the 1 μM NE superfusion (Fig. 2a, Table 1). NE increased the input resistances of the IPSP-SPNs, but not to a significant degree. The EPSPs and IPSPs also increased during the superfusion of NE, but again, the effect was less than significant. The amplitudes of the EPSPs and IPSPs also tended to increase after NE. At 5 min after the NE washout, the membrane potential recovered to −43.8 ± 3.2 mV (Fig. 2a, b-1, b-2) (Table 1).
Responses of IPSP-SPNs to NE and Dxm. a IPSP-SPNs were similarly depolarized with EPSP-SPN from 3 to 5 min after the commencement of the superfusion with 1 μM NE. The responses recovered in about 10 min after washout. Dxm brought about no change of the membrane potential. b-1 IPSPs in a faster sweep representation at control. b-2 Increase of IPSPs by NE. The application of NE increased the frequency and augmented the voltage of the IPSPs. b-3 Decrease of IPSPs by Dxm. Dxm decreased both the frequency and voltage of the IPSPs
Next, we examined the effects of Dxm on IPSP-SPNs after NE. Dxm brought about no significant change of the IPSP-SPNs membrane potential compared with that after the NE washout (−38.2 ± 3.1 mV vs. −44.0 ± 3.2 mV). Dxm decreased both the numbers and amplitudes of the EPSPs and IPSPs (Fig. 2b-3) (Table 1).
Effects of noradrenaline and dexmedetomidine hydrochloride on firing-SPNs
We next examined the effects of NE on the19 firing-SPNs. Of the 19 firing-SPNs, 12 were superfused with NE by a similar method. NE depolarized 9 of 12 firing-SPNs (−45.5 ± 3.8 mV vs. −40.8 ± 4.2 mV, P < 0.05) (Fig. 3a). NE also increased the input resistances of the IPSP-SPNs, but not to a significant degree (284.8 ± 164.7 MΩ vs. 292.4 ± 185.5 MΩ). The frequency of firing also increased during the superfusion of NE (0.57 ± 0.06 Hz vs. 1.47 ± 0.39 Hz, P < 0.05). At 5 min after the NE washout, the membrane potential recovered to −44.0 ± 4.1 mV. Dxm brought about no significant change of the membrane potential of the firing-SPNs compared with that after the NE washout (−44.0 ± 4.1 mV vs. −44.6 ± 4.0 mV). Dxm decreased the numbers of firing (1.47 ± 0.39 Hz vs. 0.24 ± 0.38 Hz, P < 0.0001, n = 9). In 3 of the 12 firing-SPNs that superfused with NE, NE induced hyperpolarization from 5 min after the commencement of NE superfusion (−44.3 ± 3.5 mV vs. −50.5 ± 4.8 mV, P < 0.05) and completely inhibited the appearance of APs (Fig. 3b). No change of the input resistances in response to the NE superfusion were observed in those 3 firing-SPNs (275.0 ± 62.0 MΩ vs. 278.5 ± 56.7 MΩ). EPSPs and IPSPs were both frequently recognized after the NE superfusion, as the disappearance of the APs made both easily viewable. After the NE washout, the membrane potential recovered and the APs reappeared (Fig. 3b). An examination of the effects of Dxm on 2 of the 3 firing neurons in which NE induced hyperpolarization revealed no significant findings of firing (2.08 ± 1.15 Hz vs. 2.20 ± 1.13 Hz) (Fig. 3b, c-1, c-2).
Responses of a firing-SPN to NE in the absence of TTX. a The firing-SPN was depolarized by NE from 3 to 4 min after the superfusion of 1 μM NE. These responses recovered in 5 min after the washout of NE. b The firing-SPN was hyperpolarized by NE from 3 to 4 min after the superfusion of 1 μM NE. These responses recovered in 5 min after the washout of NE. c-1 A faster sweep representation of action potentials at control. c-2 A faster sweep representation of action potentials after Dxm
Finally, we examined the effects of NE on 7/19 firing-SPNs in the presence of TTX. Spontaneous and current-induced action potentials disappeared within 10 min after the TTX superfusion was commenced. NE depolarized 5 firing-SPNs (−45.0 ± 1.7 mV vs. −38.2 ± 3.8 mV, P < 0.05) (Fig. 4a) and hyperpolarized 2 firing-SPNs (Fig. 4b) after the TTX (−7.1 mV and –3.2 mV change).
The responses of a firing-SPN to NE in the presence of TTX. a Depolarization of the firing-SPN accompanying the generation of spontaneous (probably calcium) action potentials from 3 to 4 min after the start of the 1 μM NE. b Another firing-SPN was hyperpolarized 3–4 min after the superfusion of 1 μM NE. The application of 10 pA (in a) or 10 pA (in b) hyperpolarizing square current pulses elicits negative deflections of the baseline membrane potential in proportion to the input resistance. Note that NE application brought about an increase of input resistance in (a) and decrease of input resistance in (b)
Discussion
Our findings from this study demonstrate that NE significantly depolarizes EPSP-SPNs and IPSP-SPNs and that the washout of NE recovers the membrane potentials in both types of neurons. These effects are regarded as direct effects by NE. As indirect effects, we found the following effects. (1) NE significantly increased EPSPs in EPSP-SPNs, and Dxm significantly attenuated the NE-induced increases of the spontaneous EPSPs. (2) NE augmented EPSPs and IPSPs in IPSP-SPNs. Dxm significantly attenuated the NE-induced action. (3) In most of the firing-SPNs, NE induced a pattern of depolarization similar to that seen in the EPSP-SPNs and IPSP-SPNs. In other firing-SPNs, NE induced membrane hyperpolarization and completely inhibited the firings. These inhibitory effects of NE were not reproduced by Dxm. (4) NE in the presence of TTX either depolarized or hypolarized in firing-SPNs.
Previous reports [6, 9] have shown that noradrenergic neurons can affect SPNs via both inhibitory hyperpolarization interactions mediated by α2 receptors and excitatory depolarization interactions mediated by α1 receptors. In other reports, α2-adrenergic antagonists blocked the inhibitory effects (hyperpolarization) of NE applied into the IML micro-iontophoretically [6,7,8].
In our experiments, Dxm (α2-adrenergic agonist) elicited no significant change of the membrane potential in EPSP-SPNs or IPSP-SPNs after recovery from NE-induced depolarization. NE did, however, induced significant hyperpolarization in some of the firing-SPNs. If NE could dominantly affect the α2-adrenergic receptor but not the α1 receptor, an α2-adrenergic agonist could exert an inhibitory effect on the SPNs. In experiments by Guyenet and Cabot [7], NE elicited both inhibitory and excitatory effects on firings in white pigeon SPNs, while α2-adrenergic antagonists blocked the inhibitory effects. NE also exerted both excitatory and inhibitory effects on SPNs in the present study. The differences in the adrenergic receptor subtypes might partly explain the differences in the responses of the different types of SPNs. Several NE receptor subtypes have been identified: the alpha1 (α1)-adrenergic receptors α1A, α1B and α1D; and the α2-adrenergic receptors α2A, α2B and α2C. The density gradients of these receptors on each SPN might lead to variables of responses to NE.
Synaptic transmission from the NE or adrenergic neurons to the SPNs may play a crucial role in cardiovascular control [7, 8, 15, 16]. There are few reports, however, on the indirect effects (i.e., presynaptic effects) conferred by NE via α2-adrenergic receptors. In our study, Dxm significantly inhibited the frequency of EPSPs in EPSP-SPNs and the voltage of IPSPs in IPSP-SPNs. Action potentials are produced by weighting of the EPSP. Previous data have supported both an excitatory and an inhibitory role of NE in regulating SPN activity firings. In experiments with the renal sympathetic nerve of rats by Shi et al. [17], the intrathecal NE application produced three patterns of firing-response: inhibitory, excitatory and biphasic. Low and high concentrations of NE contrarily exerted inhibitory and excitatory effects in their experiments, but they also identified totally reverse actions on prazosin and yohimbine in other renal sympathetic nerve. The firing-SPNs in our experiments responded differently to NE than the EPSP-SPNs and IPSP-SPNs, even though the NE was administered at the same concentration. There may have been an underlying variation in the electrophysiologic characteristics of the SPNs from the outset.
Lewis et al. [18] showed both inhibitory and excitatory actions of 5-hydroxytryptamine (5-HT) on neonate rat SPNs. They concluded that the 5-HT receptor also mediated the excitatory effect for firing. The 5-HT receptor also takes part in the responses to the α2 agonist. Further studies will be needed to clarify these points. Miyazaki et al. [13] demonstrated that NE inhibited the excitatory postsynaptic currents evoked by electrical stimulation. They concluded that the activation of presynaptic α2 receptors on SPNs decreased glutamate release. Kamisaki et al. [19] found that NE regulated endogenous glutamate release via α2 receptors. These results are consistent with our observations of EPSP-SPNs, specially the inhibition of the EPSPs by Dxm. Dxm may inhibit EPSPs by suppression of glutamate release through α2 receptors in EPSP-SPNs. Deuchars et al. [20] used the whole-cell patch-clamp technique to examine the responses of SPNs to stimulation of the rostral ventrolateral medulla (RVLM). The stimulation of the RVLM evoked EPSPs in all of the identified SPNs and evoked IPSPs in most of other SPNs. Deuchars et al. and others [21, 22] support that GABAergic RVLM neurons innervate SPNs in the thoracic spinal cord as a long descending monosynaptic inhibitory pathway. Our results therefore suggest the presence of GABAergic spinal interneurons that send inhibitory synaptic inputs to the SPNs. The IPSP-SPNs showed tonic IPSPs in our study. These tonic IPSPs were derived from the releases of GABA as synaptic transmissions [23,24,25,26]. The mechanisms of the inhibitory effects of IPSPs by Dxm on IPSP-SPNs may be associated with the release of GABA. Presynaptic glycine released from terminal synapses can also elicit IPSPs [27]. The mechanisms underlying this IPSP induction via presynaptic glycine release from terminal synapses await further study.
Conclusions
Our results suggest that the SPNs receive inhibitory modulation through adrenergic receptors. Some SPNs can be directly inhibited via effects independent of the α2 receptors.
References
Feng G, Yang Y, Chen J, Wu Z, Zheng Y, Li W, Dai W, Guan P, Zhong C. Ranolazine attenuated heightened plasma norepinephrine and B-type natriuretic peptide-45 in improving cardiac function in rats with chronic ischemic heart failure. Am J Transl Res. 2016;8:1295–301.
Marques FZ, Eikelis N, Bayles RG, Lambert EA, Straznicky NE, Hering D, Esler MD, Head GA, Barton DA, Schlaich MP, Lambert GW. A polymorphism in the norepinephrine transporter gene is associated with affective and cardiovascular disease through a microrna mechanism. Mol Psychiatry. 2017;22:134–41.
Fleetwood-Walker SM, Coote JH. Contribution of noradrenaline-, dopamine- and adrenaline-containing axons to the innervation of different regions of the spinal cord of the cat. Brain Res. 1981;206:95–106.
Seybold VS, Elde RP. Receptor autoradiography in thoracic spinal cord: correlation of neurotransmitter binding sites with sympathoadrenal neurons. J Neurosci. 1984;4:2533–42.
Zivin JA, Reid JL, Saavedra JM, Kopin IJ. Quantitative localization of biogenic amines in the spinal cord. Brain Res. 1975;99:293–301.
Coote JH, Lewis DI. Bulbospinal catecholamine neurones and sympathetic pattern generation. J Physiol Pharmacol. 1995;46:259–71.
Guyenet PG, Cabot JB. Inhibition of sympathetic preganglionic neurons by catecholamines and clonidine: mediation by an alpha-adrenergic receptor. J Neurosci. 1981;1:908–17.
Kadzielawa K. Inhibition of the activity of sympathetic preganglionic neurones and neurones activated by visceral afferents, by alpha-methylnoradrenaline and endogenous catecholamines. Neuropharmacology. 1983;22:3–17.
Nishi S, Yoshimura M, Polosa C. Organization of the Autonomic Nervous System: Central and Peripheral Mechanisms. New York: Liss; 1987.
Minoura Y, Onimaru H, Iigaya K, Homma I, Kobayashi Y. Electrophysiological responses of sympathetic preganglionic neurons to Ang II and aldosterone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R699–706.
Tanabe A, Onimaru H, Suzuki H, Takeyama Y, Homma I. Effects of corticotropin-releasing factor on intermediolateral cell column neurons of newborn rats. Auton Neurosci Basic Clin. 2012;171:36–40.
Tani M, Yazawa I, Ikeda K, Kawakami K, Onimaru H. Long-lasting facilitation of respiratory rhythm by treatment with TRPA1 agonist, cinnamaldehyde. J Neurophysiol. 2015;114:989–98.
Miyazaki T, Kobayashi H, Tosaka T. Presynaptic inhibition by noradrenaline of the EPSC evoked in neonatal rat sympathetic preganglionic neurons. Brain Res. 1998;790:170–7.
Tsuzawa K, Minoura Y, Takeda S, Inagaki K, Onimaru H. Effects of alpha2-adorenoceptor agonist dexmedetomidine on respiratory rhythm generation of newborn rats. Neurosci Lett. 2015;597:117–20.
Weidmann P, Beretta-Piccoli C, Link L, Bianchetti MG, Boehringer K, Morton JJ. Cardiovascular counterregulation during sympathetic inhibition in normal subjects and patients with mild hypertension. Hypertension. 1983;5:873–80.
Coote JH, Macleod VH, Fleetwood-Walker S, Gilbey MP. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981;215:135–45.
Shi H, Lewis DI, Coote JH. Effects of activating spinal alpha-adrenoreceptors on sympathetic nerve activity in the rat. J Auton Nerv Syst. 1988;23:69–78.
Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurones in the neonate rat spinal cord in vitro. Brain Res. 1993;610:267–75.
Kamisaki Y, Hamada T, Maeda K, Ishimura M, Itoh T. Presynaptic alpha 2 adrenoceptors inhibit glutamate release from rat spinal cord synaptosomes. J Neurochem. 1993;60:522–6.
Deuchars SA, Spyer KM, Gilbey MP. Stimulation within the rostral ventrolateral medulla can evoke monosynaptic gabaergic IPSPs in sympathetic preganglionic neurons in vitro. J Neurophysiol. 1997;77:229–35.
Miura M, Takayama K, Okada J. Distribution of glutamate- and GABA-immunoreactive neurons projecting to the cardioacceleratory center of the intermediolateral nucleus of the thoracic cord of SHR and WKY rats: a double-labeling study. Brain Res. 1994;638:139–50.
Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. J Physiol. 1994;478(Pt 3):423–35.
Wang L, Spary E, Deuchars J, Deuchars SA. Tonic gabaergic inhibition of sympathetic preganglionic neurons: a novel substrate for sympathetic control. J Neurosci. 2008;28:12445–52.
Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–50.
Kaneda K, Kita H. Synaptically released gaba activates both pre- and postsynaptic GABA(B) receptors in the rat globus pallidus. J Neurophysiol. 2005;94:1104–14.
Purves D, Augustine G, Fitzpatrick D. Excitatory and inhibitory postsynaptic potentials. Neuroscience. 2001; 2nd ed.
Jeong HJ, Jang IS, Moorhouse AJ, Akaike N. Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J Physiol. 2003;550:373–83.
Acknowledgements
Support for this study was provided by JSPS KAKENHI Grant Number 16K09455.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Minoura, Y., Onimaru, H., Iigaya, K. et al. Modulation of sympathetic preganglionic neuron activity via adrenergic receptors. Hypertens Res 41, 499–505 (2018). https://doi.org/10.1038/s41440-018-0049-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0049-x
- Springer Nature Singapore Pte Ltd.