Abstract
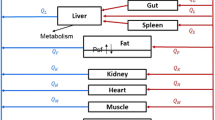
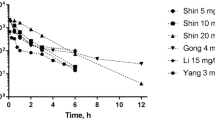
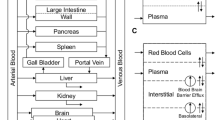
Topotecan is a chemotherapeutic agent of choice for the second-line treatment of recurrent ovarian cancer. In this article, we have developed a physiologically based pharmacokinetic model to characterize and predict topotecan concentrations in mouse plasma and tissues. Single intravenous (IV) doses (5, 10 and 30 mg/kg) of topotecan were administered to male Swiss Webster mice, with plasma and tissue samples collected over 24 h, and with sample analysis by high performance liquid chromatography. Topotecan disposition in the lungs, heart, muscle, skin, spleen, gut, liver, brain and adipose was described by perfusion rate-limited compartments, whereas the testes and intraperitoneal (IP) fluid were described with permeability rate-limited compartments. The kidneys were modeled as a permeability rate-limited compartment with nonlinear efflux. The model included enterohepatic recycling of topotecan, with re-absorption of drug secreted in the bile and nonlinear bioavailability. Topotecan demonstrated dose-dependent, nonlinear pharmacokinetics and its elimination was described by nonlinear clearance from the liver and a parallel nonlinear and linear clearance from the kidneys. Mean tissue-to-plasma partition coefficients ranged from 0.123 (brain) to 55.3 (kidney). The model adequately characterized topotecan pharmacokinetics in plasma and tissue for all three doses. Additionally, the model provided good prediction of topotecan pharmacokinetics from several external data sets, including prediction of topotecan tissue pharmacokinetics following administration of 1 or 20 mg/kg IV, and prediction of plasma pharmacokinetics following doses of 1, 1.25, 15, 20 and 80 mg/kg IV and 20 mg/kg IP.







Similar content being viewed by others
References
Markman M (1997) Topotecan: an important new drug in the management of ovarian cancer. Semin Oncol 24(1 Suppl 5):S1–S5
Anand A, Chan SY (2004) The use of topotecan for relapsed ovarian cancer in accordance with the National Institute for Clinical Excellence guidance 2001: the Nottingham experience. Clin Oncol (R Coll Radiol) 16(8):543–548
Herben VM, ten Bokkel Huinink WW, Beijnen JH (1996) Clinical pharmacokinetics of topotecan. Clin Pharmacokinet 31(2):85–102
Takimoto CH, Arbuck SG (1997) Clinical status and optimal use of topotecan. Oncology (Williston Park) 11(11):1635–1646
Brogden RN, Wiseman LR (1998) Topotecan. A review of its potential in advanced ovarian cancer. Drugs 56(4):709–723
Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C (1999) Topotecan—a novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology 56(1):1–12
Zamboni WC, Houghton PJ, Johnson RK, Hulstein JL, Crom WR, Cheshire PJ, Hanna SK, Richmond LB, Luo X, Stewart CF (1998) Probenecid alters topotecan systemic and renal disposition by inhibiting renal tubular secretion. J Pharmacol Exp Ther 284(1):89–94
Guichard S, Montazeri A, Chatelut E, Hennebelle I, Bugat R, Canal P (2001) Schedule-dependent activity of topotecan in ovcar-3 ovarian carcinoma xenograft: pharmacokinetic and pharmacodynamic evaluation. Clin Cancer Res 7(10):3222–3228
van Warmerdam LJ, ten Bokkel Huinink WW, Rodenhuis S, Koier I, Davies BE, Rosing H, Maes RA, Beijnen JH (1995) Phase I clinical and pharmacokinetic study of topotecan administered by a 24-hour continuous infusion. J Clin Oncol 13(7):1768–1776
Chen J, Lu Q, Balthasar JP (2007) Mathematical modeling of topotecan pharmacokinetics and toxicodynamics in mice. J Pharmacokinet Pharmacodyn 34(6):829–847
Herben VM, Schoemaker E, Rosing H, van Zomeren DM, ten Bokkel Huinink WW, Dubbelman R, Hearn S, Schellens JH, Beijnen JH (2002) Urinary and fecal excretion of topotecan in patients with malignant solid tumours. Cancer Chemother Pharmacol 50(1):59–64
Rosing H, Herben VM, van Gortel-van Zomeren DM, Hop E, Kettenes-van den Bosch JJ, ten Bokkel Huinink WW, Beijnen JH (1997) Isolation and structural confirmation of n-desmethyl topotecan, a metabolite of topotecan. Cancer Chemother Pharmacol 39(6):498–504
Rosing H, van Zomeren DM, Doyle E, Bult A, Beijnen JH (1998) O-glucuronidation, a newly identified metabolic pathway for topotecan and n-desmethyl topotecan. Anticancer Drugs 9(7):587–592
Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24(17):7612–7621
de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13(21):6440–6449
Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH (2000) Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 92(20):1651–1656
Hendricks CB, Rowinsky EK, Grochow LB, Donehower RC, Kaufmann SH (1992) Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue. Cancer Res 52(8):2268–2278
Leger F, Loos WJ, Fourcade J, Bugat R, Goffinet M, Mathijssen RH, Verweij J, Sparreboom A, Chatelut E (2004) Factors affecting pharmacokinetic variability of oral topotecan: a population analysis. Br J Cancer 90(2):343–347
Chen J, Balthasar JP (2005) High-performance liquid chromatographic assay for the determination of total and free topotecan in the presence and absence of anti-topotecan antibodies in mouse plasma. J Chromatogr B 816(1–2):183–192
Nedelman JR, Gibiansky E, Lau DT (1995) Applying bailer’s method for AUC confidence intervals to sparse sampling. Pharm Res 12(1):124–128
Holder DJ (2001) Comments on nedelman and jia’s extension of satterthwaite’s approximation applied to pharmacokinetics. J Biopharm Stat 11(1–2):75–79
De Cesare M, Zunino F, Pace S, Pisano C, Pratesi G (2000) Efficacy and toxicity profile of oral topotecan in a panel of human tumour xenografts. Eur J Cancer 36(12):1558–1564
Fujita H, Okamoto M, Takao A, Abe H, Ishii R, Arase H (1995) Pharmacokinetics of SK & F 104864 in experimental animals. II. Tissue distribution. Gan To Kagaku Ryoho 22(12):1789–1792
Balthasar J, Fung HL (1994) Utilization of antidrug antibody fragments for the optimization of intraperitoneal drug therapy: studies using digoxin as a model drug. J Pharmacol Exp Ther 268(2):734–739
Balthasar JP, Fung HL (1996) Inverse targeting of peritoneal tumors: selective alteration of the disposition of methotrexate through the use of anti-methotrexate antibodies and antibody fragments. J Pharm Sci 85(10):1035–1043
O’Dwyer PJ, LaCreta FP, Haas NB, Halbherr T, Frucht H, Goosenberg E, Yao KS (1994) Clinical, pharmacokinetic and biological studies of topotecan. Cancer Chemother Pharmacol 34(Suppl):S46–S52
Grochow LB, Rowinsky EK, Johnson R, Ludeman S, Kaufmann SH, McCabe FL, Smith BR, Hurowitz L, DeLisa A, Donehower RC et al (1992) Pharmacokinetics and pharmacodynamics of topotecan in patients with advanced cancer. Drug Metab Dispos 20(5):706–713
Strel’tsov SA, Mikheikin AL, Grokhovskii SL, Oleinikov VA, Kudelina IA, Zhuze AL (2002) Interaction of topotecan, DNA topoisomerase I inhibitor, with double-stranded polydeoxyribonucleotides. 4. Topotecan binds preferably to the GC base pairs of DNA. Mol Biol (Mosk) 36(5):912–930
Zhuang Y, Fraga CH, Hubbard KE, Hagedorn N, Panetta JC, Waters CM, Stewart CF (2006) Topotecan central nervous system penetration is altered by a tyrosine kinase inhibitor. Cancer Res 66(23):11305–11313
Bischoff KB, Dedrick RL, Zaharko DS, Longstreth JA (1971) Methotrexate pharmacokinetics. J Pharm Sci 60(8):1128–1133
Yamagata T, Kusuhara H, Morishita M, Takayama K, Benameur H, Sugiyama Y (2007) Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab Dispos 35(7):1142–1148
Fenneteau F, Turgeon J, Couture L, Michaud V, Li J, Nekka F (2009) Assessing drug distribution in tissues expressing P-glycoprotein through physiologically based pharmacokinetic modeling: model structure and parameters determination. Theor Biol Med Model 6(2):1–13
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55(20):4611–4622
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13(4):407–484
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10(7):1093–1095
Fennell TR, Brown CD (2001) A physiologically based pharmacokinetic model for ethylene oxide in mouse, rat, and human. Toxicol Appl Pharmacol 173(3):161–175
Gearhart JM, Jepson GW, Clewell HJ, Andersen ME, Conolly RB (1994) Physiologically based pharmacokinetic model for the inhibition of acetylcholinesterase by organophosphate esters. Environ Health Perspect 102(Suppl 11):51–60
Acknowledgment
This work was supported by grant CA118213 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, D.K., Balthasar, J.P. Physiologically based pharmacokinetic model for topotecan in mice. J Pharmacokinet Pharmacodyn 38, 121–142 (2011). https://doi.org/10.1007/s10928-010-9181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-010-9181-1