Abstract
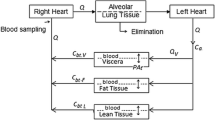
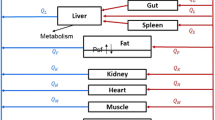
The objective was to develop a physiologically-based pharmacokinetic (PBPK) model to characterize the whole-body disposition of paclitaxel (formulated in Cremophor EL and ethanol—Taxol®) in mice and to evaluate the utility of this model for predicting pharmacokinetics in other species. Published studies that reported paclitaxel plasma and tissue concentration–time data following single intravenous bolus administration of Taxol® to mice were used; and the PBPK model included plasma, liver, lungs, kidneys, spleen, heart, gastrointestinal tract, and remainder compartments. The final model resulted in a good description of the experimental plasma and tissues data in mice, where all tissues were represented by a single compartment, except the remainder that included two sub-compartments. The predictive performance of the PBPK model was assessed by evaluating its utility in predicting pharmacokinetics of paclitaxel in rats and humans. The relationship between species body weights (mice, rats, rabbits, and humans) and plasma clearance was determined by power-based regression, and resulting allometric exponent was 0.86. The model demonstrated reasonable predictions of plasma and tissue paclitaxel concentration–time profiles in rats and plasma profiles in humans. The proposed PBPK model represents an important basis that can be further utilized for characterization of novel formulations of paclitaxel.







Similar content being viewed by others
References
Rowinsky EK, Donehower RC (1993) The clinical pharmacology of paclitaxel (Taxol). Semin Oncol 20(4 Suppl 3):16–25
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93(9):2325–2327
Rowinsky EK, Cazenave LA, Donehower RC (1990) Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst 82(15):1247–1259
Schiff PB, Horwitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 77(3):1561–1565
Hamel E, del Campo AA, Lowe MC, Lin CM (1981) Interactions of taxol, microtubule-associated proteins, and guanine nucleotides in tubulin polymerization. J Biol Chem 256(22):11887–11894
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277(5698):665–667
Wang TH, Wang HS, Soong YK (2000) Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer 88(11):2619–2628
Tarr BD, Yalkowsky SH (1987) A new parenteral vehicle for the administration of some poorly water soluble anti-cancer drugs. J Parenter Sci Technol 41(1):31–33
Swindell CS, Krauss NE, Horwitz SB, Ringel I (1991) Biologically active taxol analogues with deleted A-ring side chain substituents and variable C-2′ configurations. J Med Chem 34(3):1176–1184
Ringel I, Horwitz SB (1991) Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 83(4):288–291
Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ (1992) Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem 35(1):145–151
Lexi-Comp, Inc. Accessed 29 Jan 2017
Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F (2000) Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res 887(2):239–249
Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J, Rowen E, Yu J, Kerr RO (2010) Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest 28(2):186–194. https://doi.org/10.3109/07357900903179591
Jing S, Qiang Z (2015) Advances in the formulation and delivery technology of paclitaxel for injection. J Chin Pharm Sci. https://doi.org/10.5246/jcps.2015.08.063
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23(31):7794–7803. https://doi.org/10.1200/JCO.2005.04.937
Wiernik PH, Schwartz EL, Einzig A, Strauman JJ, Lipton RB, Dutcher JP (1987) Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol 5(8):1232–1239. https://doi.org/10.1200/jco.1987.5.8.1232
Rizzo J, Riley C, von Hoff D, Kuhn J, Phillips J, Brown T (1990) Analysis of anticancer drugs in biological fluids: determination of taxol with application to clinical pharmacokinetics. J Pharm Biomed Anal 8(2):159–164
Walle T, Walle UK, Kumar GN, Bhalla KN (1995) Taxol metabolism and disposition in cancer patients. Drug Metab Dispos 23(4):506–512
Gianni L, Kearns CM, Capri G, Vigano L, Locatelli A, Bonadonna G, Egorin MJ (1995) Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13(1):180–190
Karlsson MO, Monlnar V, Freijs A, Nygren P, Bergh J, Larsson R (1999) Pharmacokinetic models for the saturable distribution of paclitaxel. Drug Metab Dispos 27(10):1220–1223
Henningsson A, Karlsson MO, Vigano L, Gianni L, Verweij J, Sparreboom A (2001) Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol 19(20):4065–4073
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73. https://doi.org/10.1146/annurev-pharmtox-010510-100540
Shin BS, Kim HJ, Hong SH, Lee JB, Hwang SW, Lee MH, Yoo SD (2009) Enhanced absorption and tissue distribution of paclitaxel following oral administration of DHP 107, a novel mucoadhesive lipid dosage form. Cancer Chemother Pharmacol 64(1):87–94. https://doi.org/10.1007/s00280-008-0849-9
Gong C, Xie Y, Wu Q, Wang Y, Deng S, Xiong D, Liu L, Xiang M, Qian Z, Wei Y (2012) Improving anti-tumor activity with polymeric micelles entrapping paclitaxel in pulmonary carcinoma. Nanoscale 4(19):6004–6017. https://doi.org/10.1039/c2nr31517c
Li S, Su Z, Sun M, Xiao Y, Cao F, Huang A, Li H, Ping Q, Zhang C (2012) An arginine derivative contained nanostructure lipid carriers with pH-sensitive membranolytic capability for lysosomolytic anti-cancer drug delivery. Int J Pharm 436(1–2):248–257. https://doi.org/10.1016/j.ijpharm.2012.06.040
Yang XY, Li YX, Li M, Zhang L, Feng LX, Zhang N (2013) Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett 334(2):338–345. https://doi.org/10.1016/j.canlet.2012.07.002
Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J (2013) PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 34(1):196–208. https://doi.org/10.1016/j.biomaterials.2012.09.044
Wang Y, Wang C, Gong C, Wang Y, Guo G, Luo F, Qian Z (2012) Polysorbate 80 coated poly (varepsilon-caprolactone)-poly (ethylene glycol)-poly (varepsilon-caprolactone) micelles for paclitaxel delivery. Int J Pharm 434(1–2):1–8. https://doi.org/10.1016/j.ijpharm.2012.05.015
Zhang W, Shi Y, Chen Y, Yu S, Hao J, Luo J, Sha X, Fang X (2010) Enhanced antitumor efficacy by paclitaxel-loaded pluronic P123/F127 mixed micelles against non-small cell lung cancer based on passive tumor targeting and modulation of drug resistance. Eur J Pharm Biopharm 75(3):341–353. https://doi.org/10.1016/j.ejpb.2010.04.017
Han LM, Guo J, Zhang LJ, Wang QS, Fang XL (2006) Pharmacokinetics and biodistribution of polymeric micelles of paclitaxel with Pluronic P123. Acta Pharmacol Sin 27(6):747–753. https://doi.org/10.1111/j.1745-7254.2006.00340.x
Tong X, Zhou J, Tan Y (2006) Liquid chromatography/tandem triple-quadrupole mass spectrometry for determination of paclitaxel in rat tissues. Rapid Commun Mass Spectrom 20(12):1905–1912. https://doi.org/10.1002/rcm.2525
Wang Y, Li Y, Wang Q, Wu J, Fang X (2008) Pharmacokinetics and biodistribution of pacliatxel-loaded pluronic P105/L101 mixed polymeric micelles. Yakugaku Zasshi 128(6):941–950
Peltier S, Oger JM, Lagarce F, Couet W, Benoit JP (2006) Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res 23(6):1243–1250. https://doi.org/10.1007/s11095-006-0022-2
Lee SW, Chang DH, Shim MS, Kim BO, Kim SO, Seo MH (2007) Ionically fixed polymeric nanoparticles as a novel drug carrier. Pharm Res 24(8):1508–1516. https://doi.org/10.1007/s11095-007-9269-5
Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, Kim DD (2007) Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J Control Release 120(3):169–177. https://doi.org/10.1016/j.jconrel.2007.05.011
Forrest ML, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM (2008) Paclitaxel prodrugs with sustained release and high solubility in poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxicity. Pharm Res 25(1):194–206. https://doi.org/10.1007/s11095-007-9451-9
Nornoo AO, Chow DS (2008) Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm 349(1–2):117–123. https://doi.org/10.1016/j.ijpharm.2007.07.043
Zhang Z, Lee SH, Gan CW, Feng SS (2008) In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm Res 25(8):1925–1935. https://doi.org/10.1007/s11095-008-9611-6
Zhang C, Qu G, Sun Y, Wu X, Yao Z, Guo Q, Ding Q, Yuan S, Shen Z, Ping Q, Zhou H (2008) Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 29(9):1233–1241. https://doi.org/10.1016/j.biomaterials.2007.11.029
Gelderblom H, Verweij J, van Zomeren DM, Ouwens L, Nooter K, Stoter G, Sparreboom A (2002) Influence of Cremophor EL on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res 8(4):1237–1241
Bulitta JB, Zhao P, Arnold RD, Kessler DR, Daifuku R, Pratt J, Luciano G, Hanauske AR, Gelderblom H, Awada A, Jusko WJ (2009) Mechanistic population pharmacokinetics of total and unbound paclitaxel for a new nanodroplet formulation versus Taxol in cancer patients. Cancer Chemother Pharmacol 63(6):1049–1063. https://doi.org/10.1007/s00280-008-0827-2
Gardner ER, Dahut W, Figg WD (2008) Quantitative determination of total and unbound paclitaxel in human plasma following Abraxane treatment. J Chromatogr B Analyt Technol Biomed Life Sci 862(1–2):213–218. https://doi.org/10.1016/j.jchromb.2007.12.013
Chen KJ, Chen WY, Chen X, Jia YM, Peng GQ, Chen L (2014) Increased elimination of paclitaxel by magnesium isoglycyrrhizinate in epithelial ovarian cancer patients treated with paclitaxel plus cisplatin: a pilot clinical study. Eur J Drug Metab Pharmacokinet 39(1):25–31. https://doi.org/10.1007/s13318-013-0136-y
Veltkamp SA, Thijssen B, Garrigue JS, Lambert G, Lallemand F, Binlich F, Huitema AD, Nuijen B, Nol A, Beijnen JH, Schellens JH (2006) A novel self-microemulsifying formulation of paclitaxel for oral administration to patients with advanced cancer. Br J Cancer 95(6):729–734. https://doi.org/10.1038/sj.bjc.6603312
Gerlowski LE, Jain RK (1983) Physiologically based pharmacokinetic modeling: principles and application. J Pharm Sci 72(10):1103–1127
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10(7):1093–1095
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13(4):407–484
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker Inc, New York
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F (1995) Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs 6 (3):339-355; 363-338
Eiseman JL, Eddington ND, Leslie J, MacAuley C, Sentz DL, Zuhowski M, Kujawa JM, Young D, Egorin MJ (1994) Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemother Pharmacol 34(6):465–471
Fetterly GJ, Straubinger RM (2003) Pharmacokinetics of paclitaxel-containing liposomes in rats. AAPS PharmSci 5(4):E32
Sparreboom A, Van Tellingen O, Nooijen WJ, Beijnen J (1998) Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs 9(1):1–17
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH (1996) Tissue distribution, metabolism and excretion of paclitaxel in mice. Anticancer Drugs 7(1):78–86
Walle T, Kumar GN, McMillan JM, Thornburg KR, Walle UK (1993) Taxol metabolism in rat hepatocytes. Biochem Pharmacol 46(9):1661–1664
Monsarrat B, Royer I, Wright M, Cresteil T (1997) Biotransformation of taxoids by human cytochromes P450: structure-activity relationship. Bull Cancer 84(2):125–133
Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijier DK, Borst P, Nooijen WJ, Beijnen JH, Van Tellingen O (1997) Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A 94(5):2031–2035
Kagan L, Gershkovich P, Wasan KM, Mager DE (2011) Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone(R)): pooled analysis of published data. AAPS J 13(2):255–264. https://doi.org/10.1208/s12248-011-9267-8
Kagan L, Gershkovich P, Wasan KM, Mager DE (2014) Dual physiologically based pharmacokinetic model of liposomal and nonliposomal amphotericin B disposition. Pharm Res 31(1):35–45. https://doi.org/10.1007/s11095-013-1127-z
Kawai R, Lemaire M, Steimer JL, Bruelisauer A, Niederberger W, Rowland M (1994) Physiologically based pharmacokinetic study on a cyclosporin derivative, SDZ IMM 125. J Pharmacokinet Biopharm 22(5):327–365
Wei Y, Xue Z, Ye Y, Wang P, Huang Y, Zhao L (2014) Pharmacokinetic and tissue distribution of paclitaxel in rabbits assayed by LC-UV after intravenous administration of its novel liposomal formulation. Biomed Chromatogr 28(2):204–212. https://doi.org/10.1002/bmc.3005
Ait-Oudhia S, Straubinger RM, Mager DE (2012) Meta-analysis of nanoparticulate paclitaxel delivery system pharmacokinetics and model prediction of associated neutropenia. Pharm Res 29(10):2833–2844. https://doi.org/10.1007/s11095-012-0775-8
TAXOL® (paclitaxel) INJECTION [package insert] (2011) Bristol-Myers Squibb Company
Rowinsky EK, Jiroutek M, Bonomi P, Johnson D, Baker SD (1999) Paclitaxel steady-state plasma concentration as a determinant of disease outcome and toxicity in lung cancer patients treated with paclitaxel and cisplatin. Clin Cancer Res 5(4):767–774
Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB (1993) Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer 68(6):1104–1109
Pushkarev VM, Starenki DV, Saenko VA, Yamashita S, Kovzun OI, Popadiuk ID, Pushkarev VV, Tronko MD (2009) Effects of low and high concentrations of antitumour drug taxol in anaplastic thyroid cancer cells. Exp Oncol 31(1):16–21
Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sorio R, Sleeboom HP, Izquierdo MA, Jodrell DI, Calvert H, Boddy AV, Hollema H, Fety R, Van der Vijgh WJ, Hempel G, Chatelut E, Karlsson M, Wilkins J, Tranchand B, Schrijvers AH, Twelves C, Beijnen JH, Schellens JH (2007) Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res 13(21):6410–6418. https://doi.org/10.1158/1078-0432.CCR-07-0064
Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC (1993) Neurotoxicity of Taxol. J Natl Cancer Inst Monogr 15:107–115
Polomano RC, Mannes AJ, Clark US, Bennett GJ (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94(3):293–304
Lee JB, Zgair A, Taha DA, Zang X, Kagan L, Kim TH, Kim MG, Yun HY, Fischer PM, Gershkovich P (2017) Quantitative analysis of lab-to-lab variability in Caco-2 permeability assays. Eur J Pharm Biopharm. https://doi.org/10.1016/j.ejpb.2016.12.027
Straubinger RM (1995) Biopharmaceutics of paclitaxel (Taxol): formulation, activity, and pharmacokinetics. In: M. S (ed) Taxol: Science and Applications, vol 22. Pharmacology and Toxicology: Basic and Clinical Aspects, illustrated edn. CRC Press, Boca Raton, p 448
Waugh WN, Trissel LA, Stella VJ (1991) Stability, compatibility, and plasticizer extraction of taxol (NSC-125973) injection diluted in infusion solutions and stored in various containers. Am J Hosp Pharm 48(7):1520–1524
Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG, Finley R (1993) Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr 15:141–147
Song D, Hsu LF, Au JL (1996) Binding of taxol to plastic and glass containers and protein under in vitro conditions. J Pharm Sci 85(1):29–31
Sonnichsen DS, Hurwitz CA, Pratt CB, Shuster JJ, Relling MV (1994) Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol 12(3):532–538. https://doi.org/10.1200/JCO.1994.12.3.532
Sparreboom A, Van Tellingen O, Nooijen WJ, Beijnen JH (1996) Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res 56(9):2112–2115
Van Tellingen O, Huizing MT, Panday VR, Schellens JH, Nooijen WJ, Beijnen JH (1999) Cremophor EL causes (pseudo-) non-linear pharmacokineitcs of paclitaxel in patients. Br J Cancer 81(2):330–335
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39(6):711–723. https://doi.org/10.1007/s10928-012-9280-2)
Lu XF, Bi K, Chen X (2016) Physiologically based pharmacokinetic model of docetaxel and interspecies scaling: comparison of simple injection with folate receptor-targeting amphiphilic copolymer-modified liposomes. Xenobiotica 46(12):1093–1104. https://doi.org/10.3109/00498254.2016.1155128
Kawai R, Mathew D, Tanaka C, Rowland M (1998) Physiologically based pharmacokinetics of cyclosporine A: extension to tissue distribution kinetics in rats and scale-up to human. J Pharmacol Exp Ther 287(2):457–468
Tang H, Mayersohn M (2006) A global examination of allometric scaling for predicting human drug clearance and the prediction of large vertical allometry. J Pharm Sci 95(8):1783–1799. https://doi.org/10.1002/jps.20481
Hu TM, Hayton WL (2001) Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci 3(4):E29
Acknowledgements
This work was supported in part by the R01 grant (CA209818) from the National Institute of Health. The authors would like to thank Dr. Luigi Brunetti, Dr. Helene Chapy, Ms. Manting Chiang, and Ms. Xizhe Gao for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following equations were used to describe the model structure for paclitaxel disposition following IV administration of Taxol:
- Plasma (pl):
-
\( V_{pl} \frac{{dC_{pl} }}{{d_{t} }} = Inf + Q_{CO} (\frac{{C_{lu} }}{{Kp_{lu} }} - C_{pl} ) \)
- Gastrointestinal tract (gi), spleen (sp), kidneys (kd), heart (hr):
-
\( V_{ti} \frac{{dC_{ti} }}{{d_{t} }} = Q_{ti} (C_{pl} - \frac{{C_{ti} }}{{Kp_{ti} }}) \)
- Liver (li):
-
\( V_{li} \frac{{dC_{li} }}{{d_{t} }} = Q_{ha} C_{pl} + Q_{sp} \frac{{C_{sp} }}{{Kp_{sp} }} + Q_{gi} \frac{{C_{gi} }}{{Kp_{gi} }} - Q_{li} \frac{{C_{li} }}{{Kp_{li} }} - CL_{li} f_{u}^{pl} \frac{{C_{li} }}{{Kp_{li} }} \)
- Lung (lu):
-
\( V_{lu} \frac{{dC_{lu} }}{{d_{t} }} = Q_{li} \frac{{C_{li} }}{{Kp_{li} }} + Q_{kd} \frac{{C_{kd} }}{{Kp_{kd} }} + Q_{hr} \frac{{C_{hr} }}{{Kp_{hr} }} + Q_{rm} C_{rm,vas} - Q_{CO} \frac{{C_{lu} }}{{Kp_{lu} }} \)
- Remainder (rm):
-
\( \begin{aligned} V_{rm,vas} \frac{{dC_{rm,vas} }}{{d_{t} }} & = Q_{rm} \left( {C_{pl} - C_{rm,vas} } \right) - PS_{rm} \left( {f_{u}^{pl} C_{rm,vas} - f_{u}^{rm} C_{rm,exv} } \right) \\ V_{rm,exv} \frac{{dC_{rm,exv} }}{{d_{t} }} & = PS_{rm} \left( {f_{u}^{pl} C_{rm,vas} - f_{u}^{rm} C_{rm,exv} } \right) \\ \end{aligned} \)
Where Inf is the infusion rate (in simulation for humans), Qti is tissue plasma flow, and Vti is tissue volume (Vrm,vas and Vrm,exv are the volumes of the vascular and extravascular subcompartments). The initial condition for plasma equation was set to \( \frac{Dose}{{V_{pl} }} \) for modeling animal data (drug was injected as an IV bolus), and was set to zero for simulating human studies in which the drug was administered as an IV infusion. The initial conditions for all other equations were set to zero.
Rights and permissions
About this article
Cite this article
Zang, X., Kagan, L. Physiologically-based modeling and interspecies prediction of paclitaxel pharmacokinetics. J Pharmacokinet Pharmacodyn 45, 577–592 (2018). https://doi.org/10.1007/s10928-018-9586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-018-9586-9