Abstract
Purpose
Lenalidomide is used widely in B-cell malignancies for its immunomodulatory activity. It is primarily eliminated via the kidneys, with a significant proportion of renal elimination attributed to active processes. Lenalidomide is a weak substrate of P-glycoprotein (P-gp), though it is unclear whether P-gp is solely responsible for lenalidomide transport. This study aimed to determine whether the current knowledge of lenalidomide was sufficient to describe the pharmacokinetics of lenalidomide in multiple tissues.
Methods
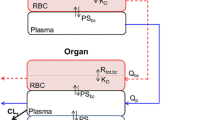
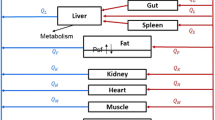
A physiologically based pharmacokinetic model was developed using the Open Systems Pharmacology Suite to explore the pharmacokinetics of lenalidomide in a variety of tissues. Data were available for mice dosed intravenously at 0.5, 1.5, 5, and 10 mg/kg, with concentrations measured in plasma, brain, heart, kidney, liver, lung, muscle, and spleen. P-gp expression and activity were sourced from the literature.
Results
The model predictions in plasma, liver, and lung were representative of the observed data (median prediction error 13%, − 10%, and 30%, respectively, with 90% confidence intervals including zero), while other tissue predictions showed sufficient similarity to the observed data. Contrary to the data, model predictions for the brain showed no drug reaching brain tissue when P-gp was expressed at the blood–brain barrier. The data were better described by basolateral transporters at the intracellular wall. Local sensitivity analysis showed that transporter activity was the most sensitive parameter in these models for exposure.
Conclusion
As P-gp transport at the blood–brain barrier did not explain the observed brain concentrations alone, there may be other transporters involved in lenalidomide disposition.





Similar content being viewed by others
References
Ghosh N, Grunwald MR, Fasan O, Bhutani M (2015) Expanding role of lenalidomide in hematologic malignancies. Cancer Manag Res 7:105–119. https://doi.org/10.2147/CMAR.S81310
Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, Mahmoudi A, Cathers B, Rychak E, Gaidarova S, Chen R, Schafer PH, Handa H, Daniel TO, Evans JF, Chopra R (2012) Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26(11):2326–2335. https://doi.org/10.1038/leu.2012.119
Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK (2011) Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 118(18):4771–4779
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R (2014) Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4 (CRBN). Br J Haematol 164(1):811–821. https://doi.org/10.1111/bjh.12708
Zhu YX, Kortuem KM, Stewart AK (2013) Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma 54(4):683–687. https://doi.org/10.3109/10428194.2012.728597
Warren KE, Goldman S, Pollack IF, Fangusaro J, Schaiquevich P, Stewart CF, Wallace D, Blaney SM, Packer R, MacDonald T, Jakacki R, Boyett JM, Kun LE (2011) Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary cns tumors: pediatric brain tumor consortium study PBTC-018. J Clin Oncol 29(3):324–329. https://doi.org/10.1200/JCO.2010.31.3601
Berg SL, Cairo MS, Russell H, Ayello J, Ingle AM, Lau H, Chen N, Adamson PC, Blaney SM (2011) Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a children’s oncology group phase I consortium report. J Clin Oncol 29(3):316–323. https://doi.org/10.1200/JCO.2010.30.8387
Drappatz J, Wong ET, Schiff D, Kesari S, Batchelor TT, Doherty L, Lafrankie DC, Ramakrishna N, Weiss S, Smith ST, Ciampa A, Zimmerman J, Ostrowsky L, David K, Norden A, Barron L, Sceppa C, Black PM, Wen PY (2009) A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 73(1):222–227. https://doi.org/10.1016/j.ijrobp.2008.03.046
Fine HA, Kim L, Albert PS, Duic JP, Ma H, Zhang W, Tohnya T, Figg WD, Royce C (2007) A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Am Assoc Cancer Res 13(23):7101–7106. https://doi.org/10.1158/1078-0432.ccr-07-1546
Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS (2006) Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol 24(34):5343–5349. https://doi.org/10.1200/jco.2005.05.0401
Foucar K, McKenna RW, Frizzera G, Brunning RD (1982) Bone marrow and blood involvement by lymphoma in relationship to the lukes—collins classification. Cancer 49(5):888–897. https://doi.org/10.1002/1097-0142(19820301)49:5%3c888:AID-CNCR11%3e3.0.CO;2-K
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78(1):21–33. https://doi.org/10.4065/78.1.21
Rozman C, Montserrat E (1995) Chronic lymphocytic leukemia. N Engl J Med 333(16):1052–1057. https://doi.org/10.1056/nejm199510193331606
Dankbar B, Padró T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE, Kienast J (2000) Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 95(8):2630–2636
Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC (2001) Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia 15(12):1950–1961
Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC (2000) Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 96(9):2943–2950
Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, Lee S, Liu Z, Garr CL, Hade EM, Ji J, Schaaf LJ, Benson DM Jr, Kraut EH, Hicks WJ, Chan KK, Chen CS, Farag SS, Grever MR, Byrd JC, Phelps MA (2011) Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein. J Clin Oncol 29(25):3427–3434. https://doi.org/10.1200/jco.2010.32.4962
Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, Garzon R, Walker A, Chandler JC, Whitman SP, Curfman J, Liu S, Schaaf L, Mickle J, Kefauver C, Devine SM, Grever MR, Marcucci G, Byrd JC (2010) Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol 28(33):4919–4925. https://doi.org/10.1200/jco.2010.30.3339
Maddocks K, Ruppert AS, Browning R, Jones J, Flynn J, Kefauver C, Gao Y, Jiang Y, Rozewski DM, Poi M, Phelps MA, Harper E, Johnson AJ, Byrd JC, Andritsos LA (2014) A dose escalation feasibility study of lenalidomide for treatment of symptomatic, relapsed chronic lymphocytic leukemia. Leuk Res 38(9):1025–1029. https://doi.org/10.1016/j.leukres.2014.05.011
Maddocks K, Wei L, Rozewski D, Jiang Y, Zhao Y, Adusumilli M, Pierceall WE, Doykin C, Cardone MH, Jones JA, Flynn J, Andritsos LA, Grever MR, Byrd JC, Johnson AJ, Phelps MA, Blum KA (2015) Reduced occurrence of tumor flare with flavopiridol followed by combined flavopiridol and lenalidomide in patients with relapsed chronic lymphocytic leukemia (CLL). Am J Hematol 90(4):327–333. https://doi.org/10.1002/ajh.23946
Chen N, Ette E, Zhou S, Weiss D, Palmisano M (2013) Population pharmacokinetics and exposure-safety of lenalidomide in patients with multiple myeloma, myelodysplastic syndromes and mantle cell lymphoma. Blood 122(21):3234
Bridoux F, Chen N, Moreau S, Arnulf B, Moumas E, Abraham J, Desport E, Jaccard A, Fermand JP (2016) Pharmacokinetics, safety, and efficacy of lenalidomide plus dexamethasone in patients with multiple myeloma and renal impairment. Cancer Chemother Pharmacol 78(1):173–182. https://doi.org/10.1007/s00280-016-3068-9
Corporation C (2017) Product monograph: revlimid® lenalidomide capsules. https://media.celgene.com/content/uploads/sites/23/Revlimid-Product_Monograph_-_English_Version.pdf. Accessed 6 Mar 2017
Chen N, Wen L, Lau H, Surapaneni S, Kumar G (2012) Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother Pharmacol 69(3):789–797. https://doi.org/10.1007/s00280-011-1760-3
Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, Laskin OL (2007) Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 47(12):1466–1475. https://doi.org/10.1177/0091270007309563
Tong Z, Yerramilli U, Surapaneni S, Kumar G (2014) The interactions of lenalidomide with human uptake and efflux transporters and UDP-glucuronosyltransferase 1A1: lack of potential for drug–drug interactions. Cancer Chemother Pharmacol 73(4):869–874. https://doi.org/10.1007/s00280-014-2415-y
Kumar G, Lau H, Laskin O (2009) Lenalidomide: in vitro evaluation of the metabolism and assessment of cytochrome P450 inhibition and induction. Cancer Chemother Pharmacol 63(6):1171–1175. https://doi.org/10.1007/s00280-008-0867-7
Connarn JN, Hwang R, Gao Y, Palmisano M, Chen N (2017) Population pharmacokinetics of lenalidomide in healthy volunteers and patients with hematologic malignancies. Clin Pharmacol Drug Dev 7(5):465–473. https://doi.org/10.1002/cpdd.372
Guglieri-López B, Pérez-Pitarch A, Moes DJAR, Porta-Oltra B, Climente-Martí M, Guchelaar HJ, Merino-Sanjuán M (2017) Population pharmacokinetics of lenalidomide in multiple myeloma patients. Cancer Chemother Pharmacol 79(1):189–200. https://doi.org/10.1007/s00280-016-3228-y
Anwer S, Collings F, Trace K, Sun Y, Sternberg A (2013) Cerebrospinal fluid penetrance of lenalidomide in meningeal myeloma. Br J Haematol 162(2):281–282. https://doi.org/10.1111/bjh.12351
Muscal JA, Sun Y, Nuchtern JG, Dauser RC, McGuffey LH, Gibson BW, Berg SL (2012) Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol 69(4):943–947. https://doi.org/10.1007/s00280-011-1781-y
Rozewski DM, Herman SEM, Towns WH, Mahoney E, Stefanovski MR, Shin JD, Yang X, Gao Y, Li X, Jarjoura D, Byrd JC, Johnson AJ, Phelps MA (2012) Pharmacokinetics and tissue disposition of lenalidomide in mice. AAPS J 14(4):872–882. https://doi.org/10.1208/s12248-012-9401-2
Upton RN, Foster DJR, Abuhelwa AY (2016) An introduction to physiologically-based pharmacokinetic models. Pediatr Anesth 26(11):1036–1046. https://doi.org/10.1111/pan.12995
Liu Q, Farley KL, Johnson AJ, Muthusamy N, Hofmeister CC, Blum KA, Schaaf LJ, Grever MR, Byrd JC, Dalton JT, Phelps MA (2008) Development and validation of a highly sensitive liquid chromatography/mass spectrometry method for simultaneous quantification of lenalidomide and flavopiridol in human plasma. Ther Drug Monit 30(5):620–627. https://doi.org/10.1097/FTD.0b013e318185813d
Eissing T, Kuepfer L, Becker C, Block M, Coboeken K, Gaub T, Goerlitz L, Jaeger J, Loosen R, Ludewig B, Meyer M, Niederalt C, Sevestre M, Siegmund H-U, Solodenko J, Thelen K, Telle U, Weiss W, Wendl T, Willmann S, Lippert J (2011) A computational systems biology software platform for multiscale modeling and simulation: integrating whole-body physiology, Disease biology, and molecular reaction networks. Front Physiol 2:4. https://doi.org/10.3389/fphys.2011.00004
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504. https://doi.org/10.1023/A:1012299115260
RC Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46(D1):D1074–D1082. https://doi.org/10.1093/nar/gkx1037
DrugBank (2017) Lenalidomide. https://www.drugbank.ca/drugs/DB00480. Accessed 12 Sept 2017
Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B (2014) A comparative encyclopedia of DNA elements in the mouse genome. Nature 515(7527):355–364. https://doi.org/10.1038/nature13992
Ferguson CS, Tyndale RF (2011) Cytochromes P450 in the brain: emerging evidence for biological significance. Trends Pharmacol Sci 32(12):708–714. https://doi.org/10.1016/j.tips.2011.08.005
Zhou S, Li Y, Kestell P, Schafer P, Chan E, Paxton JW (2005) Transport of thalidomide by the human intestinal caco-2 monolayers. Eur J Drug Metab Pharmacokinet 30(1–2):49–61
Ball K, Bouzom F, Scherrmann J-M, Walther B, Declèves X (2013) Physiologically based pharmacokinetic modelling of drug penetration across the blood-brain barrier–towards a mechanistic IVIVE-based approach. AAPS J 15(4):913–932. https://doi.org/10.1208/s12248-013-9496-0
Chowdhury G, Shibata N, Yamazaki H, Guengerich FP (2014) Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analog of thalidomide. Chem Res Toxicol 27(1):147–156. https://doi.org/10.1021/tx4004215
Lampert I, Catovsky D, Marsh GW, Child JA, Galton DAG (1980) The histopathology of prolymphocytic leukaemia with particular reference to the spleen: a comparison with chronic lymphocytic leukaemia. Histopathology 4(1):3–19. https://doi.org/10.1111/j.1365-2559.1980.tb02893.x
Willmann S, Lippert J, Schmitt W (2005) From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol 1(1):159–168. https://doi.org/10.1517/17425255.1.1.159
Acknowledgements
The research produced was supported by an Australian Government Research Training Program Scholarship (to JHH). The authors acknowledge that the Australian Centre for Pharmacometrics is an initiative of the Australian Government as part of the National Collaborative Research Infrastructure Strategy. Additional support came from the National Institutes of Health (R01CA201382 to MAP) and an Eli Lilly Fellowship (to DMR).
Author information
Authors and Affiliations
Contributions
Participated in research design: JHH, RNU, SER, DMR, MAP, and DJRF. Conducted experiments: DMR. Contributed new reagents or analytical tools: RNU, MAP, and DJRF. Performed data analysis: JHH. Wrote or contributed to the writing of the manuscript: JHH, RNU, SER, DMR, MAP, and DJRF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hughes, J.H., Upton, R.N., Reuter, S.E. et al. Development of a physiologically based pharmacokinetic model for intravenous lenalidomide in mice. Cancer Chemother Pharmacol 84, 1073–1087 (2019). https://doi.org/10.1007/s00280-019-03941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03941-z