Abstract
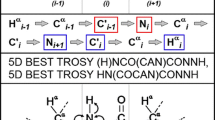
We propose a new alpha proton detection based approach for the sequential assignment of natively unfolded proteins. The proposed protocol superimposes on following features: HA-detection (1) enables assignment of natively unfolded proteins at any pH, i.e., it is not sensitive to rapid chemical exchange undergoing in natively unfolded proteins even at moderately high pH. (2) It allows straightforward assignment of proline-rich polypeptides without additional proline-customized experiments. (3) It offers more streamlined and less ambiguous assignment based on solely intraresidual 15N(i)-13C′(i)-Hα(i) (or 15N(i)-13Cα(i)-Hα(i)) and sequential 15N(i + 1)-13C′(i)-Hα(i) (or 15N(i + 1)-13Cα(i)-Hα(i)) correlation experiments together with efficient use of chemical shifts of 15N and 13C′ nuclei, which show smaller dependence on residue type. We have tested the proposed protocol on two proteins, small globular 56-residue GB1, and highly disordered, proline-rich 47-residue fifth repeat of EspFU. Using the proposed approach, we were able to assign 90% of 1Hα, 13Cα, 13C′, 15N chemical shifts in EspFU. We reckon that the HA-detection based strategy will be very useful in the assignment of natively unfolded proline-rich proteins or polypeptide chains.



Similar content being viewed by others
References
Alho N, Klaavuniemi T, Ylanne J, Permi P, Mattila S (2007) Backbone NMR assignment of the internal interaction site of ALP. Biomol NMR Assign 1:85–87
Bai Y, Milne JS, Mayne L, Englander SW (1993) Primary structure effects on peptide group hydrogen exchange. Proteins Struct Funct Genet 17:75–86
Barna JCJ, Laue ED, Mayger MR, Skilling J, Worrall SJP (1987) Exponential sampling, an alternative method for sampling in two-dimensional NMR experiments. J Magn Reson 73:69–77
Bendall MR (1995) Heteronuclear J coupling precession during spin-lock and adiabatic pulses. Use of adiabatic inversion pulses in high-resolution NMR. J Magn Reson A116:46–58
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006) 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Reson Spectr 48:25–45
Bermel W, Bertini I, Csizmok V, Felli IC, Pierattelli R, Tompa P (2009) H-start for exclusively heteronuclear NMR spectroscopy: the case of intrinsically disordered protein. J Magn Reson 198:275–281
Bottomley MJ, Macias MJ, Liu Z, Sattler M (1999) A novel NMR experiment for the sequential assignment of proline residues and proline stretches in 13C/15N-labeled proteins. J Biomol NMR 13:381–385
Brutscher B (2002) Intraresidue HNCA and COHNCA experiments for protein backbone resonance assignment. J Magn Reson 156:155–159
Cheng HC, Skehan BM, Campellone KG, Leong JM, Rosen MK (2008) Structural mechanism of WASP activation by the enterohaemorrhagic E. coli effector EspF(U). Nature 454:1009–1013
Coggins BE, Zhou P (2008) High resolution 4-D spectroscopy with sparse concentric shell sampling and FFT-CLEAN. J Biomol NMR 42:225–239
Delaglio F, Torchia DA, Bax A (1991) Measurement of 15N–13C J couplings in staphylococcal nuclease. J Biomol NMR 1:439–446
Dyson HJ, Wright PE (2001) Nuclear magnetic resonance methods for elucidation of structure and dynamics in disordered states. Methods Enzymol 339:258–270
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Freeman R, Kupče E (2004) Distant echoes of the accordion: reduced dimensionality, GFT-NMR, and projection-reconstruction of multidimensional spectra. Concepts Magn Reson 23A:63–75
Goddard TD, Kneller DG (2004) SPARKY 3. University of California, San Francisco
Grzesiek S, Bax A, Hu J-S, Kaufman J, Palmer I, Stahl SJ, Tjandra N, Wingfield PT (1997) Refined solution structure and backbone dynamics of HIV-1 Nef. Prot Sci 6:1248–1263
Hu K, Vögeli B, Clore GM (2007) Spin-state selective carbon-detected HNCO with TROSY optimization in all dimensions and double echo-antiecho sensitivity enhancement in both indirect dimensions. J Am Chem Soc 129:5484–5491
Jaravine VA, Zhuravleva AV, Permi P, Ibraghimov I, Orekhov VY (2008) Hyperdimensional NMR spectroscopy with nonlinear sampling. J Am Chem Soc 130:3927–3936
Jiang L, Coggins BE, Zhou P (2005) Rapid assignment of protein side chain resonances using projection-reconstruction of (4, 3)D HC(CCO)NH and intra-HC(C)NH experiments. J Magn Reson 175:170–176
Kanelis V, Donaldson L, Muhandiram DR, Rotin D, Forman-Kay JD, Kay LE (2000) Sequential assignment of proline-rich regions in proteins: application to modular binding domain complexes. J Biomol NMR 16:253–259
Kay LE, Ikura M, Tschudin R, Bax A (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson 89:496–514
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kazimierczuk K, Zawadzka A, Koźmiński V, Zhukov I (2006) Random sampling of evolution time space and Fourier transform processing. J Biomol NMR 36:157–168
Kupće E, Wagner G (1995) Wideband homonuclear decoupling in protein spectra. J Magn Reson 109A:329–333
Mäntylahti S, Tossavainen H, Hellman M, Permi P (2009) An intraresidual i(HCA)CO(CA)NH experiment for the assignment of main-chain resonances in 15N, 13C labeled proteins. J Biomol NMR 45:301–310
Marion D (2006) Processing of ND NMR spectra sampled in polar coordinates: a simple Fourier transform instead of a reconstruction. J Biomol NMR 36:45–54
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR-spectra without phase cycling–application to the study of hydrogen-exchange in proteins. J Magn Reson 85:393–399
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Nietlispach D (2004) A selective intra-HN(CA)CO experiment for the backbone assignment of deuterated proteins. J Biomol NMR 28:131–136
Nietlispach D, Ito Y, Laue ED (2002) A novel approach for the sequential backbone assignment of larger proteins: selective intra-HNCA and DQ-HNCA. J Am Chem Soc 124:11199–11207
Permi P (2002) Intraresidual HNCA: an experiment for correlating only intraresidual backbone resonances. J Biomol NMR 23:201–209
Permi P, Annila A (2004) Coherence transfer in proteins. Prog Nucl Magn Reson Spectr 44:97–137
Pervushin K, Riek R, Wider G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A 94:12366–12731
Powers R, Gronenborn AM, Clore GM, Bax A (1991) Three-dimensional triple-resonance NMR of 13C/15N-enriched proteins using constant-time evolution. J Magn Reson 94:209–213
Rovnyak D, Frueh DP, Sastry M, Sun Z-YJ, Stern AS, Hoch JC, Wagner G (2004) Accelerated acquisition of high resolution triple-resonance spectra using non-uniform sampling and maximum entropy reconstruction. J Magn Reson 170:15–21
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectr 34:93–158
Sayers EW, Torchia DA (2001) Use of the carbonyl chemical shift to relieve degeneracies in triple-resonance assignment experiments. J Magn Reson 153:246–253
Schleucher J, Schwendinger MG, Sattler M, Schmidt P, Glaser SJ, Sørensen OW, Griesinger C (1994) A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed-field gradients. J Biomol NMR 4:301–306
Shaka AJ (1985) Composite pulses for ultra-broadband spin inversion. Chem Phys Lett 120:201–205
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broad-band decoupling–Waltz-16. J Magn Reson 52:335–338
Silver MS, Joseph RJ, Hoult DI (1984) Highly selective π/2 and π pulse generation. J Magn Reson 59:347–351
Tompa P (2002) Intrinsically unstructured proteins. Trends Biochem Sci 27:527–533
Tossavainen H, Permi P (2004) Optimized pathway selection in intraresidual triple-resonance experiments. J Magn Reson 170:244–251
Varian Inc. NMR Systems (2006) Varian NMR Systems with VNMRJ Software
Wang AC, Bax A (1995) Reparametrization of the Karplus relation for 3 J(Hα–N) and 3 J(HN–C′) in peptides from uniformly 13C/15N-enriched human ubiquitin. J Am Chem Soc 117:1810–1813
Wang A, Grzesiek S, Tschudin R, Lodi PJ, Bax A (1995) Sequential backbone assignment of isotopically enriched proteins in D20 by deuterium-decoupled HA(CA)N and HA(CACO)N. J Biomol NMR 5:376–382
Yamazaki T, Lee W, Arrowsmith CH, Muhandiram DR, Kay LE (1994) A suite of triple-resonance NMR experiments for the backbone assignment of 15N, 13C, 2H labeled proteins with high-sensitivity. J Am Chem Soc 116:11655–11666
Yao J, Dyson HJ, Wright PE (1997) Chemical shift dispersion and secondary structure prediction in unfolded and partly folded proteins. FEBS Lett 419:285–289
Acknowledgments
This work was financially supported by the grants 122170 and 131144 (to P. P.) from the Academy of Finland. We thank Vytautas Raulinaitis for useful discussions, and Elina Ahovuo and Jessica Buchmüller for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mäntylahti, S., Aitio, O., Hellman, M. et al. HA-detected experiments for the backbone assignment of intrinsically disordered proteins. J Biomol NMR 47, 171–181 (2010). https://doi.org/10.1007/s10858-010-9421-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-010-9421-0