Abstract
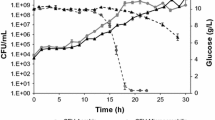
Three bacterial (Pedobacter heparinus, Pedobacter piscium, Pedobacter cryoconitis) and three yeast strains (Saccharomyces cerevisiae, Leucosporidiella creatinivora, Rhodotorula glacialis) of different thermal classes (mesophiles and psychrophiles) were tested for the effect of temperature on a range of growth parameters, including optical density, viable cell numbers, and cell dry mass, in order to determine the temperature conditions under which maximum biomass formation is obtained. Maximum values of growth parameters obtained at the stationary growth phase of the strains were used for statistical calculation. Temperature had a significant (P ≤ 0.05) effect on all growth parameters for each strain; correlations between the growth parameters were significant (P ≤ 0.05–0.01). The maximum growth temperature or the temperature at which microbial growth was fastest was in no case the temperature at which the investigated strains produced the highest amount of biomass. All tested psychrophilic bacteria and yeast strains produced highest amounts of cells (as calculated per mg cell dry mass or per OD600 unit) at 1°C, while cell numbers of mesophiles were highest at 20°C. Thus, cultivation temperatures close to the maximum growth temperature are not appropriate for studying psychrophiles.


Similar content being viewed by others
References
Bakermans C, Nealson KH (2004) Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobactcer cryopegella. J Bacteriol 186:2340–2345
Bergauer P, Fonteyne PA, Nolard N, Schinner F, Margesin R (2005) Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts. Chemosphere 59:909–918
Buchon L, Laurent P, Gounot AM, Guespin-Michel J (2000) Temperature dependence of extracellular enzymes production by psychrotrophic and psychrophilic bacteria. Biotechnol Lett 22:1577–1581
Chablain PA, Philippe G, Groboillot A, Truffaut N, Guespin-Michel J (1979) Isolation of a soil psychrotrophic toluene-degrading Pseudomonas strain: influence of temperature on the growth characteristics on different substrates. Res Microbiol 148:153–161
D’Amico S, Collins T, Marx JC, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389
Feller G (2007) Life at low temperatures: is disorder the driving force? Extremophiles 11:211–216
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208
Feller G, Narinx E, Arpigny JL, Zekhnini Z, Swings J, Gerday C (1994) Temperature dependence of growth, enzyme secretion and activity of psychrophilic Antarctic bacteria. Appl Microbiol Biotechnol 41:477–479
Feller G, Narinx E, Arpigny JL, Aittaleb M, Baise E, Genicot S, Gerday C (1996) Enzymes from psychrophilic organisms. FEMS Microbiol Rev 18:189–202
Gerday C (2008) On life in the cold: approximations and mistakes. In: Program and abstracts, 3rd international conference on polar and alpine microbiology, Banff, Canada, May 11–15, 2008, p 24
Glansdorff N, Xu Y (2002) Microbial life at low temperatures: mechanisms of adaptation and extreme biotopes. Implications for exobiology and the origin of life. Recent Res Dev Microbiol 6:1–21
Guillou C, Guespin-Michel JF (1996) Evidence for two domains of growth temperature for the psychrotrophic bacterium Pseudomonas fluorescens MF0. Appl Environ Microbiol 62:3319–3324
Gounot AM, Russell NJ (1999) Physiology of cold-adapted microorganisms. In: Margesin R, Schinner F (eds) Cold-adapted organisms. Springer, Berlin, pp 33–55
Huston AL, Krieger-Brockett BB, Deming JW (2000) Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environ Microbiol 2:383–388
Jaouen T, De E, Chevalier S, Orange N (2004) Size dependence on growth temperature is a common characteristic of the major outer membrane protein OprF in psychrotrophic and mesophilic Pseudomonas species. Appl Environ Microbiol 70:6665–6669
Kato T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2001) Isolation and characterization of psychrotrophic bacteria from oil-reservoir water and oil sands. Appl Microbiol Biotechnol 55:794–800
Koch AL (1994) Growth measurement. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. ASM Press, Washington DC, pp 248–292
Margesin R, Schinner F (1992) Extracellular protease production by psychrotrophic bacteria from glaciers. Int Biodeterior Biodegradation 29:177–189
Margesin R, Gander S, Zacke G, Gounot AM, Schinner F (2003a) Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 7:451–458
Margesin R, Spröer C, Schumann P, Schinner F (2003b) Pedobacter cryoconitis sp. nov., a novel facultatively psychrophilic bacterium from alpine glacier cryoconite. Int J Syst Evol Microbiol 53:1291–1296
Margesin R, Fonteyne PA, Redl B (2005a) Low-temperature biodegradation of high amounts of phenol by Rhodococcus spp. and basidiomycetous yeasts. Res Microbiol 156:68–75
Margesin R, Fauster V, Fonteyne PA (2005b) Characterization of cold-active pectate lyases from psychrophilic Mrakia frigida. Lett Appl Microbiol 40:453–459
Margesin R, Fonteyne PA, Schinner F, Sampaio JP (2007) Rhodotorula psychrophila sp. nov., Rhodotorula psychrophenolica sp. nov. and Rhodotorula glacialis sp. nov., novel psychrophilic basidiomycetous yeast species from alpine environments. Int J Syst Evol Microbiol 57:2179–2184
Margesin R, Schinner F, Marx JC, Gerday C (eds) (2008) Psychrophiles from biodiversity to biotechnology. Springer, Berlin, p 462
Panikov NS, Sizova MV (2007) Growth kinetics of microorganisms isolated from Alaskan soil and permafrost in soild media frozen down to −35°C. FEMS Microbiol Ecol 59:500–512
Russell NJ (1990) Cold adaptation of microorganisms. Phil Trans R Soc Lond B 329:595–611
Sampaio JP, Gadanho M, Bauer R, Weisz M (2003) Taxonomic studies in the Microbotryomycetidae: Leudosporidium golubevii sp.nov., Leudosporidiella gen.nov. and the new orders Leucosporidiales and Sporodiobolales. Mycol Prog 2:53–68
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Ann Rev Biochem 75:403–433
Steyn PL, Seger P, Vancanneyt M, Kersters K, Joubert JJ (1998) Classification of heparinolytic bacteria into a new genus, Pedobacter, comprising four species: Pedobacter heparinus comb nov., Pedobacter piscium comb. nov., Pedobacter africanus sp. nov. and Pedobacter saltans sp. nov. Proposal of the family Sphingobacteriaceae fam. nov. Int J Syst Microbiol 48:165–177
Acknowledgments
The author thanks Karin Weber for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Matsunaga.
Rights and permissions
About this article
Cite this article
Margesin, R. Effect of temperature on growth parameters of psychrophilic bacteria and yeasts. Extremophiles 13, 257–262 (2009). https://doi.org/10.1007/s00792-008-0213-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0213-3