Abstract
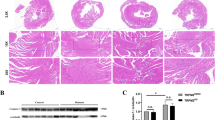
Increased reactive oxygen species (ROS) are traditionally viewed as arising from the metabolic flux of diabetes, although reduction in the activity of anti-oxidant systems has also been implicated. Among the latter is the major thiol reducing thioredoxin system, the activity of which may be diminished by high glucose-induced expression of its endogenous inhibitor, thioredoxin interacting protein (TxnIP). We assessed TxnIP mRNA/protein expression along with thioredoxin activity in human right atrial biopsy specimens from subjects with and without diabetes undergoing coronary artery grafting. In correlative experimental studies, we examined TxnIP expression in both type 1 and type 2 rodent models of diabetic cardiomyopathy. Finally, we used in vitro gene silencing to determine the contribution of changes in TxnIP abundance to the high glucose-induced reduction in thioredoxin activity. In human right atrial biopsies, diabetes was associated with a >30-fold increase in TxnIP gene expression and a 17 % increase in TxnIP protein expression (both p < 0.05). This was associated with a 21 % reduction in thioredoxin activity when compared to human non-diabetic cardiac biopsy samples (all p < 0.05). In correlative animal studies, both type 1 and type 2 diabetic rats demonstrated a significant increase in TxnIP mRNA and reduction in thioredoxin activity when compared to non-diabetic animals (all p < 0.05). This was associated with a significant increase in ROS (p < 0.05 when compared with control). In cultured cardiac myocytes, high glucose increased ROS and TxnIP mRNA expression, in association with a reduction in thioredoxin activity (p < 0.01). These findings were abrogated by TxnIP small interfering RNA (siRNA). Scrambled siRNA had no effect upon ROS or TxnIP expression. High glucose reduces thioredoxin activity and increases ROS via TxnIP overexpression. These findings suggest that impaired thiol reductive capacity, through altered TxnIP expression, contributes to increased ROS in the diabetic heart.






Similar content being viewed by others
Abbreviations
- ATCC:
-
American type culture collection
- ANOVA:
-
Analysis of variance
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- EDP:
-
End-diastolic pressure
- EDPVR:
-
The slope of the end-diastolic pressure volume relationship
- EDV:
-
End-diastolic volume
- ESP:
-
End systolic pressure
- FBS:
-
Fetal bovine serum
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- OCT:
-
Optimal cutting temperature
- PCR:
-
Polymerase chain reaction
- PLSD:
-
Fishers protected least significant difference
- PRSW:
-
The slope of the preload recruitable stroke work relationship
- PV:
-
Left ventricular pressure–volume loop
- RAS:
-
Renin-angiotensin system
- Ren-2:
-
TGR(mRen-2)27 transgenic (Ren-2) rats
- RVU:
-
Relative volume unit
- ROS:
-
Reactive oxygen species
- SiRNA:
-
Small interfering RNA
- STZ:
-
Streptozotocin
- SW:
-
Stroke work
- TRX:
-
Thioredoxin
- TxnIP:
-
Thioredoxin interacting protein
References
Alter DA, Khaykin Y, Austin PC et al (2003) Processes and outcomes of care for diabetic acute myocardial infarction patients in Ontario: do physicians undertreat? Diabetes Care 26:1427–1434. doi:10.2337/diacare.26.5.1427
Uchmanowicz I, Loboz-Grudzien K, Jankowska-Polanska B, Sokalski L (2011) Influence of diabetes on health-related quality of life results in patients with acute coronary syndrome treated with coronary angioplasty. Acta Diabetol 50:217–225. doi:10.1007/s00592-011-0280-2
Donahoe SM, Stewart GC, McCabe CH et al (2007) Diabetes and mortality following acute coronary syndromes. JAMA 298:765–775. doi:10.1001/jama.298.7.765
Woodfield SL, Lundergan CF, Reiner JS et al (1996) Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. JAC 28:1661–1669. doi:10.1016/S0735-1097(96)00397-X
Gao F, Zhou YJ, Shen H et al (2012) Meta-analysis of percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with diabetes and left main and/or multivessel coronary artery disease. Acta Diabetol 50:765–773. doi:10.1007/s00592-012-0411-4
Farkouh ME, Domanski M, Sleeper LA et al (2012) Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 367:2375–2384. doi:10.1056/NEJMoa1211585
Tarquini R, Lazzeri C, Pala L et al (2010) The diabetic cardiomyopathy. Acta Diabetol 48:173–181. doi:10.1007/s00592-010-0180-x
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820. doi:10.1038/414813a
Berlett BS (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316. doi:10.1074/jbc.272.33.20313
Yamawaki H, Berk BC (2005) Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens 14:149
Emiroglu MY, Esen ÖB, Bulut M et al (2010) GGT levels in type II diabetic patients with acute coronary syndrome (does diabetes have any effect on GGT levels in acute coronary syndrome?). Acta Diabetol 50:21–25. doi:10.1007/s00592-010-0208-2
Chen K-S, DeLuca HF (1994) Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta (BBA)-Gene Struct Expr 1219:26–32. doi:10.1016/0167-4781(94)90242-9
Xiang G, Seki T, Schuster MD et al (2005) Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem 280:39394–39402. doi:10.1074/jbc.M502966200
Cheng DW, Jiang Y, Shalev A et al (2006) An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem 112:189–218. doi:10.1080/13813450601093518
Hirota T, Okano T, Kokame K et al (2002) Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J Biol Chem 277:44244–44251. doi:10.1074/jbc.M206233200
Kobayashi T, Uehara S, Ikeda T et al (2003) Vitamin D3 up-regulated protein-1 regulates collagen expression in mesangial cells. Kidney Int 64:1632–1642. doi:10.1046/j.1523-1755.2003.00263.x
Qi W, X Chen, Gilbert RE et al (2007) High glucose-induced thioredoxin-interacting protein in renal proximal tubule cells is independent of transforming growth factor-β1. Am J Pathol 171:744–754. doi:10.2353/ajpath.2007.060813
Schulze PC, Yoshioka J, Takahashi T et al (2004) Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279:30369–30374. doi:10.1074/jbc.M400549200
Advani A, Gilbert RE, Thai K et al (2009) Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol 20:730–741. doi:10.1681/ASN.2008020142
Advani A, Kelly DJ, Cox AJ et al (2009) The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54:261–269. doi:10.1161/HYPERTENSIONAHA.109.128645
Connelly KA, Advani A, Kim S et al (2011) The cardiac (pro)renin receptor is primarily expressed in myocyte transverse tubules and is increased in experimental diabetic cardiomyopathy. J Hypertens 29:1175–1184. doi:10.1097/HJH.0b013e3283462674
Holmgren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Meth Enzymol 252:199–208
Connelly KA, Kelly DJ, Zhang Y et al (2007) Functional, structural and molecular aspects of diastolic heart failure in the diabetic (mRen-2)27 rat. Cardiovasc Res 76:280–291. doi:10.1016/j.cardiores.2007.06.022
Kuliszewski M, Kabir G, Fujii H et al (2011) 724 Characterisation of a novel rodent model of diabetes induced heart failure with preserved ejection fraction—the Goto Kakizaki rat. Can J Cardiol 27:S328. doi:10.1016/j.cjca.2011.07.598
Vahtola E, Louhelainen M, Merasto S et al (2008) Forkhead class O transcription factor 3a activation and Sirtuin1 overexpression in the hypertrophied myocardium of the diabetic Goto–Kakizaki rat. J Hypertens 26:334–344. doi:10.1097/HJH.0b013e3282f293c8
Connelly KA, Prior DL, Kelly DJ et al (2006) Load-sensitive measures may overestimate global systolic function in the presence of left ventricular hypertrophy: a comparison with load-insensitive measures. Am J Physiol Heart Circ Physiol 290:H1699–H1705. doi:10.1152/ajpheart.00577.2005
Georgakopoulos D, Mitzner WA, Chen CH et al (1998) In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol 274:H1416–H1422
Pacher P, Nagayama T, Mukhopadhyay P et al (2008) Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3:1422–1434. doi:10.1038/nprot.2008.138
Yang B, Larson DF, Beischel J et al (2001) Validation of conductance catheter system for quantification of murine pressure-volume loops. J Invest Surg 14:341–355. doi:10.1080/089419301753435710
Kelly DJ, Zhang Y, Hepper C et al (2003) Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 52:512–518
Kai H, Muraishi A, Sugiu Y et al (1998) Expression of proto-oncogenes and gene mutation of sarcomeric proteins in patients with hypertrophic cardiomyopathy. Circ Res 83:594–601
Brownlee M (2005) The pathobiology of diabetic complications a unifying mechanism. Diabetes 54:1615–1625
Berndt C, Lillig CH, Holmgren A (2006) Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol 292:H1227–H1236. doi:10.1152/ajpheart.01162.2006
Nishiyama A, Matsui M, Iwata S et al (1999) Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 274:21645–21650
Junn E, Han SH, Im JY et al (2000) Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164:6287–6295
Chen J, Cha-Molstad H, Szabo A, Shalev A (2009) Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 296:E1133–E1139. doi:10.1152/ajpendo.90944.2008
Turoczi T, Chang VW-H, Engelman RM et al (2003) Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. J Mol Cell Cardiol 35:695–704
Tanito M, Masutani H, Nakamura H et al (2002) Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest Ophthalmol Vis Sci 43:1162–1167
Minn AH, Hafele C, Shalev A (2005) Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 146:2397–2405. doi:10.1210/en.2004-1378
Hui STY, Andres AM, Miller AK et al (2008) Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci USA 105:3921–3926. doi:10.1073/pnas.0800293105
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223. doi:10.1161/CIRCULATIONAHA.106.679597
Yoshioka J, Chutkow WA, Lee S et al (2012) Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest 122:267–279. doi:10.1172/JCI44927DS1
Li X, Rong Y, Zhang M et al (2009) Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun 381:660–665. doi:10.1016/j.bbrc.2009.02.132
Ma L, Robinson LN, Towle HC (2006) ChREBP*Mlx Is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 281:28721–28730. doi:10.1074/jbc.M601576200
Acknowledgments
These studies were supported by a HSF Grant-in-aid #000484, #7201 and a Grant from the J. P Bickell Foundation. Dr. Kim Connelly was supported by a HSF Canada Phase 1 clinician scientist award. Dr. Richard Gilbert is the Canada Research Chair in Diabetes Complications and this study was supported, in part, by the Canada Research Chair Program. Dr. Advani is supported by a Canadian Diabetes Association Clinician Scientist Award. Imaging for this study work was carried out at the BioImaging Facility of St. Michael’s Hospital, Toronto, Canada.
Conflict of interest
Kim A Connelly, Andrew Advani, Suzanne L. Advani, Yuan Zhang, Young M. Kim, Vanessa Shen, Kerri Thai, Darren J. Kelly and Richard E. Gilbert declare that they have no conflict of interest.
Human and Animal Rights
All animal studies were approved by the St Michael’s Animal ethics care committee in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Informed Consent
Informed consent was obtained from all patients for being included in the study in accordance with the requirements of the St Michael’s Hospital human research ethics committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Connelly, K.A., Advani, A., Advani, S.L. et al. Impaired cardiac anti-oxidant activity in diabetes: human and correlative experimental studies. Acta Diabetol 51, 771–782 (2014). https://doi.org/10.1007/s00592-014-0608-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0608-9