Abstract
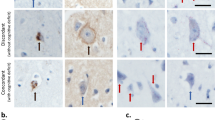
Phosphorylated Smad2/3 (pSmad2/3), the central mediators of transforming growth factor (TGF)-beta signaling, were recently identified in tau-positive inclusions in certain neurodegenerative disorders. To clarify whether the localization of pSmad2/3 is altered in amyotrophic lateral sclerosis (ALS), we immunohistochemically examined spinal cords from sporadic ALS (SALS), from familial ALS (FALS) patients with the A4V mutation in their Cu/Zn superoxide dismutase (SOD1) gene, and from G93A mutant SOD1 transgenic (mSOD1 Tg) mice. In control spinal cords, pSmad2/3 immunoreactivity was observed exclusively in neuronal and glial nuclei. In SALS and FALS patients the nuclei showed increased immunoreactivity for pSmad2/3. Noticeably, round hyaline inclusions (RHIs) and skein-like inclusions of SALS patients were immunoreactive for pSmad2/3. Double immunofluorescence staining for pSmad2/3 and transactive response-DNA-binding protein (TDP)-43 revealed co-localization of these proteins within RHIs. In contrast, Bunina bodies in SALS and Lewy body-like hyaline inclusions (LBHIs) in FALS were devoid of labeling for pSmad2/3. Similarly, in the mSOD1 Tg mice pSmad2/3 immunoreactivity was increased in the nuclei, while LBHIs were not labeled. These findings suggest increased TGF-beta-Smad signaling in SALS, FALS, and mSOD1 Tg mice, as well as impaired TGF-beta signal transduction in RHI-bearing neurons of SALS patients, presumably at the step of pSmad2/3 translocation into the nucleus. The pathomechanisms, including the process of inclusion development, appears to be different between SALS and mSOD1-related FALS or Tg mice.



Similar content being viewed by others
References
Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL (1997) TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem 272:27678–27685
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Brionne TC, Tesseur I, Masliah E, Wyss-Coray T (2003) Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40:1133–1145
Brooks BR (1994) El Escorial world federation of neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 124 [Suppl]:96–107
Chalmers KA, Love S (2007) Neurofibrillary tangles may interfere with Smad 2/3 signaling in neurons. J Neuropathol Exp Neurol 66:158–167
Chalmers KA, Love S (2007) Phosphorylated Smad 2/3 colocalizes with phospho-tau inclusions in Pick disease, progressive supranuclear palsy, and corticobasal degeneration but not with alpha-synuclein inclusions in multiple system atrophy or dementia with Lewy bodies. J Neuropathol Exp Neurol 66:1019–1026
Day WA, Koishi K, Nukuda H, McLennan IS (2005) Transforming growth factor-beta 2 causes an acute improvement in the motor performance of transgenic ALS mice. Neurobiol Dis 19:323–330
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79
Flanders KC, Ren RF, Lippa CF (1998) Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol 54:71–85
Henrich-Noack P, Prehn JH, Krieglstein J (1994) Neuroprotective effects of TGF-beta 1. J Neural Transm Suppl 43:33–45
Hirano A, Kurland LT, Sayre GP (1967) Familial amyotrophic lateral sclerosis: a subgroup characterized by posterior and spinocerebellar tract involvement and hyaline inclusions in the anterior horn cells. Arch Neurol 16:232–243
Houi K, Kobayashi T, Kato S, Mochio S, Inoue K (2002) Increased plasma TGF-beta 1 in patients with amyotrophic lateral sclerosis. Acta Neurol Scand 106:299–301
Iłzecka J, Stelmasiak Z, Dobosz B (2002) Transforming growth factor-beta 1 (TGF-beta 1) in patients with amyotrophic lateral sclerosis. Cytokine 20:239–243
Kurisaki A, Kose S, Yoneda Y, Heldin CH, Moustakas A (2001) Transforming growth factor-beta induces nuclear import of Smad3 in an importin-beta1 and Ran-dependent manner. Mol Biol Cell 12:1079–1091
Lee HG, Ueda M, Zhu X, Perry G, Smith MA (2006) Ectopic expression of phospho-Smad2 in Alzheimer’s disease: uncoupling of the transforming growth factor-beta pathway? J Neurosci Res 84:1856–1861
Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH Jr (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Schmierer B, Hill CS (2007) TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8:970–982
Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS (2006) Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol 65:45–54
Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542
Ten Dijke P, Hill CS (2004) New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29:265–273
Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T (2006) Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest 116:3060–3069
Ueberham U, Ueberham E, Gruschka H, Arendt T (2006) Altered subcellular location of phosphorylated Smads in Alzheimer’s disease. Eur J Neurosci 24:2327–2334
Wate R, Ito H, Zhang JH, Ohnishi S, Nakano S, Kusaka H (2005) Expression of an endoplasmic reticulum-resident chaperone, glucose-regulated stress protein 78, in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol 110:557–562
Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L (1997) Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature 389:603–606
Xu L (2006) Regulation of Smad activities. Biochim Biophys Acta 1759:503–513
Zhang J, Ito H, Wate R, Ohnishi S, Nakano S, Kusaka H (2006) Altered distributions of nucleocytoplasmic transport-related proteins in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol 112:673–680
Acknowledgments
We express our sincere appreciation to Drs. Kengo Fujita, Yoshimi Kinoshita, and Makoto Nishii, as well as to Miss Tomoko Takemi, for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Ito, H., Wate, R. et al. Phosphorylated Smad2/3 immunoreactivity in sporadic and familial amyotrophic lateral sclerosis and its mouse model. Acta Neuropathol 115, 327–334 (2008). https://doi.org/10.1007/s00401-007-0337-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0337-z