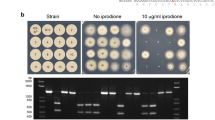
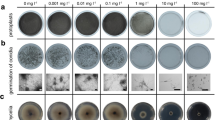
Abstract
Dominant selectable markers are beneficial for transformation of many fungi, particularly those model species where repeated transformations may be required. A carboxin resistance allele of the Coprinopsis cinerea sdi1 gene, encoding the iron-sulphur protein subunit of succinate dehydrogenase, was developed by introducing a suitable point mutation in the histidine block responsible for binding of the associated iron ion. This modified gene was used successfully to confer carboxin resistance upon transformation of C. cinerea protoplasts. Plasmids previously used to establish hygromycin transformation systems of several basidiomycete species, such as pAN7-1 and phph004, failed to give rise to hygromycin-resistant transformants of C. cinerea, whilst pPHT1 was successful. Sequencing of these constructs showed that the hygromycin resistance gene in pAN7-1 and phph004 had been modified removing the codons encoding two lysine residues following the N-terminal methionine. Replacement of the deleted 6 bp (AAA AAG) in the truncated hph gene led to generation of hygromycin-resistant transformants indicating the importance of these two codons for expression in C. cinerea. Phleomycin-resistant (ble) transformants were also obtained, but only with the intron-containing construct pblei004, showing that an intron is necessary to obtain phleomycin-resistant C. cinerea. This contrasts with hygromycin-resistance, where introns are not required for expression, emphasising the variability in importance of these elements.




Similar content being viewed by others
References
Bartholomew K, Dos Santos G, Dumonceaux T, Charles T, Archibald F (2001) Genetic transformation of Trametes versicolor to phleomycin resistance with the dominant selectable marker shble. Appl Microbiol Biotechnol 56:201–214
Binninger DM, Skrzynia C, Pukkila PJ, Casselton LA (1987) DNA-mediated transformation of the basidiomycete Coprinus cinereus. Embo J 6:835–840
Binninger DM, Lechevanton L, Skrzynia C, Shubkin CD, Pukkila PJ (1991) Targeted transformation in Coprinus cinereus. Mol Gen Genet 227:245–251
Broomfield PL, Hargreaves JA (1992) A single amino-acid change in the iron-sulphur protein subunit of succinate dehydrogenase confers resistance to carboxin in Ustilago maydis. Curr Genet 22:117–121
Burns C, Gregory KE, Kirby M, Cheung MK, Riquelme M, Elliott TJ, Challen MP, Bailey A, Foster GD (2005) Efficient GFP expression in the mushrooms Agaricus bisporus and Coprinus cinereus requires introns. Fungal Genet Biol 42:191–199
Burns C, Leach KM, Elliott TJ, Challen MP, Foster GD, Bailey A (2006) Evaluation of Agrobacterium-mediated transformation of Agaricus bisporus using a range of promoters linked to hygromycin resistance. Mol Biotechnol 32:129–138
Challen MP, Gregory KE, Sreenivasaprasad S, Rogers CC, Cutler SB, Diaper DC, Elliot TJ, Foster GD (2000) Transformation technologies for mushrooms. Mushroom Sci 15:165–172
Chen X, Stone M, Schlagnhaufer C, Romaine CP (2000) A fruiting body tissue method for efficient Agrobacterium-mediated transformation of Agaricus bisporus. Appl Environ Microbiol 66:4510–4513
Combier JP, Melayah D, Raffier C, Gay G, Marmeisse R (2003) Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in the symbiotic ectomycorrhizal fungus Hebeloma cylindrosporum. FEMS Microbiol Lett 220:141–148
Cummings WJ, Celerin M, Crodian J, Brunick LK, Zolan ME (1999) Insertional mutagenesis in Coprinus cinereus: use of a dominant selectable marker to generate tagged, sporulation-defective mutants. Curr Genet 36:371–382
Godio RP, Fouces R, Gudina EJ, Martin JF (2004) Agrobacterium tumefaciens-mediated transformation of the antitumor clavaric acid-producing basidiomycete Hypholoma sublateritium. Curr Genet 46:287–294
Granado JD, Kertesz-Chaloupková K, Aebi M, Kües U (1997) Restriction enzyme-mediated DNA integration in Coprinus cinereus. Mol Gen Genet 256:28–36
Gritz L, Davies J (1983) Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 25:179–188
Hanif M, Pardo AG, Gorfer M, Raudaskoski M (2002) T-DNA transfer and integration in the ectomycorrhizal fungus Suillus bovinus using hygromycin B as a selectable marker. Curr Genet 41:183–188
Heneghan MN, Costa AM, Challen MP, Mills PR, Bailey A, Foster GD (2007) A comparison of methods for successful triggering of gene silencing in Coprinus cinereus. Mol Biotechnol 35:283–296
Honda Y, Matsuyama T, Irie T, Watanabe T, Kuwahara M (2000) Carboxin resistance transformation of the homobasidiomycete fungus Pleurotus ostreatus. Curr Genet 37:209–212
Irie T, Sato T, Saito K, Honda Y, Watanabe T, Kuwahara M, Enei H (2003) Construction of a homologous selectable marker gene for Lentinula edodes transformation. Biosci Biotechnol Biochem 67:2006–2009
Ito Y, Muraguchi H, Seshime Y, Oita S, Yanagi SO (2004) Flutolanil and carboxin resistance in Coprinus cinereus conferred by a mutation in the cytochrome b560 subunit of succinate dehydrogenase complex (Complex II). Mol Genet Genomics 272:328–335
James TY, Boulianne RP, Bottoli APF, Granado JD, Aebi M, Kües U (2002) The pab1 gene of Coprinus cinereus encodes a bifunctional protein for para-aminobenzoic acid (PABA) synthesis: implications for the evolution of fused PABA synthases. J Basic Microbiol 42:91–103
Kemppainen M, Circosta A, Tagu D, Martin F, Pardo AG (2005) Agrobacterium-mediated transformation of the ectomycorrhizal symbiont Laccaria bicolor S238N. Mycorrhiza 16:19–22
Keon JP, White GA, Hargreaves JA (1991) Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen, Ustilago maydis. Curr Genet 19:475–481
Kertesz-Chaloupková K, Walser PJ, Granado JD, Aebi M, Kües U (1998) Blue light overrides repression of asexual sporulation by mating type genes in the basidiomycete Coprinus cinereus. Fungal Genet Biol 23:95–109
Kikuchi M, Kitamoto N, Shishido K (2004) Secretory production of Aspergillus oryzae xylanase XynF1, xynF1 cDNA product, in the basidiomycete Coprinus cinereus. Appl Microbiol Biotechnol 63:728–733
Kilaru S, Hoegger PJ, Majcherczyk A, Burns C, Shishido K, Bailey A, Foster GD, Kües U (2006) Expression of laccase gene lcc1 in Coprinopsis cinerea under control of various basidiomycetous promoters. Appl Microbiol Biotechnol 71:200–210
Kim K, Leem Y, Choi HT (2002) Transformation of the medicinal basidiomycete Trametes versicolor to hygromycin B resistance by restriction enzyme mediated integration. FEMS Microbiol Lett 209:273–276
Kües U (2000) Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Rev 64:316–353
Kües U, Richardson WV, Tymon AM, Mutasa ES, Göttgens B, Gaubatz S, Gregoriades A, Casselton LA (1992) The combination of dissimilar alleles of the Aα and Aβ gene complexes, whose proteins contain homeo domain motifs, determines sexual development in the mushroom Coprinus cinereus. Genes Dev 6:568–577
Liu D, Coloe S, Baird R, Pederson J (2000) Rapid mini-preparation of fungal DNA for PCR. J Clin Microbiol 38:471
Lugones LG, Scholtmeijer K, Klootwijk R, Wessels JGH (1999) Introns are necessary for mRNA accumulation in Schizophyllum commune. Mol Microbiol 32:681–689
Ma B, Mayfield MB, Gold MH (2001) The green fluorescent protein gene functions as a reporter of gene expression in Phanerochaete chrysosporium. Appl Environ Microbiol 67:948–955
Marmeisse R, Gay G, Debaud JC, Casselton LA (1992) Genetic transformation of the symbiotic basidiomycete fungus Hebeloma cylindrosporum. Curr Genet 22:41–45
Mattern IE, Unkles S, Kinghorn JR, Pouwels PH, van den Hondel CA (1987) Transformation of Aspergillus oryzae using the A niger pyrG gene. Mol Gen Genet 210:460–471
Mooibroek H, Kuipers AG, Sietsma JH, Punt PJ, Wessels JG (1990) Introduction of hygromycin B resistance into Schizophyllum commune: preferential methylation of donor DNA. Mol Gen Genet 222:41–48
Ogawa K, Yamazaki T, Hasebe T, Kajiwara S, Watanabe A, Asada Y, Shishido K (1998) Molecular breeding of the basidiomycete Coprinus cinereus strains with high lignin-decolorization and -degradation activities using novel heterologous protein expression vectors. Appl Microbiol Biotechnol 49:285–289
Pardo AG, Hanif M, Raudaskoski M, Gorfer M (2002) Genetic transformation of ectomycorrhizal fungi mediated by Agrobacterium tumefaciens. Mycol Res 106:132–137
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA (1987) Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124
Sambrook J, Russell DW (2001) Molecular cloning, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Scholtmeijer K, Wösten HAB, Springer J, Wessels JGH (2001) Effect of introns and AT-rich sequences on expression of the bacterial hygromycin B resistance gene in the basidiomycete Schizophyllum commune. Appl Environ Microbiol 67:481–483
Schuren FHJ, Wessels JGH (1994) Highly efficient transformation of the homobasidiomycete Schizophyllum commune to phleomycin resistance. Curr Genet 26:179–183
Skinner W, Bailey A, Renwick A, Keon J, Gurr S, Hargreaves J (1998) A single amino-acid substitution in the iron-sulphur protein subunit of succinate dehydrogenase determines resistance to carboxin in Mycosphaerella graminicola. Curr Genet 34:393–398
van de Rhee MD, Graca PMA, Huizing HJ, Mooibroek H (1996) Transformation of the cultivated mushroom, Agaricus bisporus, to hygromycin B resistance. Mol Gen Genet 250:252–258
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Kues.
Rights and permissions
About this article
Cite this article
Kilaru, S., Collins, C.M., Hartley, A.J. et al. Investigating dominant selection markers for Coprinopsis cinerea: a carboxin resistance system and re-evaluation of hygromycin and phleomycin resistance vectors. Curr Genet 55, 543–550 (2009). https://doi.org/10.1007/s00294-009-0266-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-009-0266-6