Summary
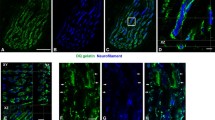
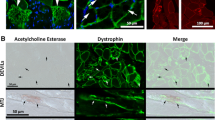
Immunocytochemistry and Western blotting were used to monitor the fate of dystrophin in the soleus muscle of the rat during a cycle of degeneration and regeneration induced by inoculation of the muscle with the venom of Notechis scutatus scutatus (the Australian tiger snake). In control muscle dystrophin was localised close to the plasma membrane. Dystrophin began to break down 3–6 h after venom inoculation, giving a characteristic discontinuous labelling pattern. At 12 h dystrophin was absent from the plasma membrane, and by 1 day the architecture of the muscle fibres had completely broken down. By 2 days post inoculation regeneration had commenced. The regenerating myofibres possessed well-organised myofibrils and the plasma membrane was intact. Dystrophin was detected by Western blot at 3 days, but was not seen in sections until regeneration of the muscle was well advanced, at 4 days post inoculation. The results suggested that although dystrophin was present in the myofibres at 3 days, it was not incorporated into the plasma membrane until 4 days post inoculation. This may be due to the influence of the functional reinnervation of the regenerating fibres, which occurs at 4–5 days, or to the growing fibres reaching a critical diameter.
Similar content being viewed by others
References
Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, Ishihara T, Nonaka I, Ozawa E, Sugita H (1988) Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 333:861–863
Arahata K, Hoffman EP, Kunkel LM, Ishiura S, Tsukahara T, Ishihara T, Sunohara T, Nonaka T, Ozawa E, Sugita H (1989) Dystrophin diagnosis: comparison of dystrophin abnormalities by immunofluorescence and immunoblotting analyses. Proc Natl Acad Sci USA 86:7154–7158
Arahata K, Ishihara T, Kamakura K, Tsukahara T, Ishiura S, Baba C, Matsumoto T, Nonaka I, Sugita H (1989) Mosaic expression of dystrophin in symptomatic carriers of Duchenne muscular dystrophy. N Engl J Med 320:138–142
Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, Kunkel LM, Hoffman EP, Rowland LP (1988) Duchenne muscular dystrophy: deficiency of dystrophin at muscle cell surface. Cell 54:447–452
Bulfield G, Sillar WG, Wight PAL, Moore KJ (1984) X-chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81:1189–1192
Byers TJ, Husain-Chishti A, Dubreuil RR, Branton D, Goldstein LSB (1989) Sequence similarity of the amino terminal of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol 109:1633–1642
Campbell KP, Kahl SD (1989) Association of dystrophin and an integral membrane glycoprotein. Nature 338:259–262
Carpenter S, Karpati G, Zubrzycka-Gaarn EE, Bulman DE, Ray PE, Worton RG (1990) Dystrophin is localised at the plasma membrane of human skeletal muscle fibres by electron microscope cytochemical study. Muscle Nerve 13:376–380
Cullen MJ, Mastaglia FL (1982) Pathological reactions of skeletal muscle. In: Mastaglia FL, Walton JN (eds) Skeletal muscle pathology. Churchill Livingstone, Edinburgh, pp 95–102
Cullen MJ, Walsh J, Nicholson LVB, Harris JB (1990) Ultrastructural localisation of dystrophin in human muscle by using gold immunolabelling. Proc R Soc Lond [B] 240:197–210
Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP (1990) Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345:315–319
Fischbeck KH (1989) The difference between Duchenne and Becker dystrophies. Neurology 39:584–585
Grubb BD, Harris JB, Schofield IS (1991) Neuromuscular transmission at newly formed neuromuscular junctions in the regenerating soleus muscle of the rat. J Physiol 441:405–421
Hagiwara Y, Yoshida M, Nonaka I, Ozawa E (1989) Developmental expression of dystrophin on the plasma membrane of rat muscle cells. Protoplasma 151:11–18
Harris JB, Cullen MJ (1990) Muscle necrosis caused by snake venoms and toxins. Electron Microsc Rev 3:183–211
Harris JB, Johnson MA (1978) Further observations on the pathological responses of rate skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol 5:587–600
Harris JB, Nicholson LVB (1990) Muscular dystrophies. Curr Op in Neurol Neurosurg 3:672–677
Harris JB, Johnson MA, Karlsson E (1975) Pathological responses of rat skeletal muscle to a single subcutaneous injection of a toxin isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol 2:383–404
Hoffman EP, Kunkel LM (1989) Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron 2:1019–1029
Hoffman EP, Brown RH, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928
Jimi T, Wakayama Y. (1990) Effect of denervation on regenerating muscle plasma membrane integrity: freeze-fracture and dystrophin immunostaining analyses. Acta Neuropathol 80:401–405
Karpati G, Pouliot Y, Zubrzycka-Gaarn EE, Carpenter S, Ray PN, Worton RG, Holland P (1989) Dystrophin is expressed in mdx skeletal muscle fibres after normal myoblast implantation. Am J Pathol 135:27–32
Koenig M, Kunkel LM (1990) Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem 265:4560–4566
Koenig M, Monaco AP, Kunkel LM (1988) The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53:219–228
Kornegay JN, Tuler SM, Miller DM, Levesque DC (1988) Muscular dystrophy in a litter of golden retriever dogs. Muscle Nerve 11:1056–1064
Maltin CA, Harris JB, Cullen MJ (1983) Regeneration of mammalian skeletal muscle following the injection of the snake-venom toxin, taipoxin. Cell Tissue Res 232:565–577
Murayama T, Sato S, Kimura S, Shimizu T, Sawada H, Maruyama K (1990) Molecular shape of dystrophin purified from rabbit skeletal muscle myofibrils. Proc Jpn Acad 66:96–99
Nicholson LVB, Johnson MA, Davison K, Barron M, Gardner-Medwin D, Bhattacharya S, Harris JB (1991). Patterns of dystrophin labelling in muscle from patients with Duchenne and Becker muscular dystrophy. In: Angelini C, Danieli GA, Fontanari D (eds) Muscular dystrophy research. Excerpta Medica 934. Elsevier. Amsterdam, pp 240–241
Nicholson LVB, Davison K, Falkous G, Harwood C, O'Donnell E, Slater CR, Harris JB (1989a). Dystrophin in skeletal muscle. I. Western blot analysis using a monoclonal antibody. J Neurol Sci 94:125–136
Nicholson LVB, Davison K, Johnson MA, Slater CR, Young C, Bhattacharya S, Gardner-Medwin D, Harris JB (1989b) Dystrophin in skeletal muscle. II. Immunoreactivity in patients with Xp21 muscular dystrophy. J Neurol Sci 94:137–146
Nicholson LVB, Johnson MA, Davies KE (1990a) Integrated dystrophin analysis using immunocytochemical, biochemical and genetic techniques. Basic Appl Histochem 34:169–175
Nicholson LVB, Johnson MA, Gardner-Medwin D, Bhattacharya S, Harris JB (1990b) Heterogeneity of dystrophin expression in patients with Duchenne and Becker muscular dystrophy. Acta Neuropathol 80:239–250
Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM (1989) Conversion of mdx myofibres from dystrophin negative to positive by injection of normal myoblasts. Nature 337:176–179
Pons F, Augier N, Heilig R, Léger J, Mornet D, Léger JJ (1990) Isolated dystrophin molecules as seen by electron microscopy. Proc Natl Acad Sci USA 87:7851–7855
Pons F, Augier N, Léger JOC, Robert A, Tomé FMS, Fardeau M, Voit T, Nicholson LVB, Mornet D, Léger JJ (1991) A homologue of dystrophin is expressed at the neuromuscular junctions of normal individuals and DMD patients, and of normal and mdx mice. Immunological evidence. FEBS Lett 282:161–165
Salviati S, Betto R, Ceoldo S, Biasia E, Bonilla E, Miranda AF, DiMauro S (1989) Cell fractionation studies indicate that dystrophin is a protein of surface membrane of skeletal muscle. Biochem J 258:837–841
Samitt CE, Bonilla E (1990) Immunocytochemical study of dystrophin at the myotendinous junction. Muscle Nerve 13:493–500
Sealock R, Butler MH, Kramarcy NR, Gao K-X, Murnane AA, Douville K, Froehner SC (1991) Localisation of dystrophin relative to acetylcholine receptor domains in electric tissue and adult and cultured skeletal muscle. J Cell Biol 113:1133–1144
Slater CR, Nicholson LVB (1991) Is dystrophin labelling always discontinuous in Becker muscular dystrophy? J Neurol Sci 101:187–192
Sugita H, Arahata K, Ishiguro T, Suhara Y, Tsukahara T, Ishiura S, Eguchi C, Nonaka I, Ozawa E (1988) Negative immunostaining of Duchenne muscular dystrophy (DMD) and mdx muscle surface membrane with antibody against systhetic peptide fragment predicted from DMD cDNA. Proc Jpn Acad 64:37–39
Watkins SC, Hoffman EP, Slayter HS, Kunkel LM (1988) Immunoelectron microscopic localisation of dystrophin in myofibres. Nature 333:863–866
Whalen RG, Harris JB, Butler-Browne GS, Sesodia S (1990) Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev Biol 141:24–40
Wolff JA, Malone RW, Williams P, Chang W, Acsadi G, Jani A, Felgner PL (1990) Direct gene transfer into mouse muscle in vivo. Science 247:1465–1468
Yoshida M, Ozawa E (1990) Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem 108:748–752
Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AHM, Belfall B, Klamut HJ, Talbot J, Hodges RS, Ray PN, Worton RG (1988) The Duchenne muscular dystrophy gene product is localised in sarcolemma of human skeletal muscle. Nature 333:466–469
Author information
Authors and Affiliations
Additional information
Supported by the Muscular Dystrophy Group of Great Britain, the Wellcome Trust, and the MRC
Rights and permissions
About this article
Cite this article
Vater, R., Cullen, M.J., Nicholson, L.V.B. et al. The fate of dystrophin during the degeneration and regeneration of the soleus muscle of the rat. Acta Neuropathol 83, 140–148 (1992). https://doi.org/10.1007/BF00308473
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00308473