Abstract
Background
Previous meta-analysis of heterogeneous surgical cohorts demonstrated reduction in postoperative pain with perioperative intravenous dexamethasone, but none have addressed adults undergoing elective abdominal surgery. The aim of this study was to determine the impact of intravenous perioperative dexamethasone on postoperative pain in adults undergoing elective abdominal surgery under general anaesthesia.
Methods
This review was prospectively registered on the international prospective register of systematic reviews (CRD42020176202). Electronic databases Medical Analysis and Retrieval System Online (MEDLINE), Exerpta Medica Database (EMBASE), (CINAHL) Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and trial registries were searched to January 28 2021 for randomised controlled trials, comparing dexamethasone to placebo or alternative antiemetic, that reported pain. The primary outcome was pain score, and secondary outcomes were time to first analgesia, opioid requirements and time to post-anaesthesia care unit (PACU) discharge.
Results
Fifty-two studies (5768 participants) were included in the meta-analysis. Pain scores ≤ 4 hour (h) were reduced in patients who received dexamethasone at rest (mean difference (MD), − 0.54, 95% confidence interval (CI) − 0.72 to − 0.35, I2 = 81%) and on movement (MD − 0.42, 95% CI − 0.62 to − 0.22, I2 = 35). In the dexamethasone group, 4–24 h pain scores were less at rest (MD − 0.31, 95% CI − 0.47 to − 0.14, I2 = 96) and on movement (MD − 0.26, 95% CI − 0.39 to − 0.13, I2 = 29) and pain scores ≥ 24 h were reduced at rest (MD − 0.38, 95% CI − 0.52 to − 0.24, I2 = 88) and on movement (MD − 0.38, 95% CI − 0.65 to − 0.11, I2 = 71). Time to first analgesia (minutes) was increased (MD 22.92, 95% CI 11.09 to 34.75, I2 = 98), opioid requirements (mg oral morphine) decreased (MD − 6.66, 95% CI − 9.38 to − 3.93, I2 = 88) and no difference in time to PACU discharge (MD − 3.82, 95% CI − 10.87 to 3.23, I2 = 59%).
Conclusions
Patients receiving dexamethasone had reduced pain scores, postoperative opioid requirements and longer time to first analgesia. Dexamethasone is an effective analgesic adjunct for patients undergoing abdominal surgery.
Similar content being viewed by others
Background
Pain is a common postoperative problem and can be associated with physical and psychological sequelae. Glucocorticoids can modify the stress response and reduce inflammation. Dexamethasone, a commonly used antiemetic, interferes with the cyclooxygenase and lipoxygenase pathways through phospholipase inhibition and has been proposed to modulate postoperative pain in surgical patients (Moore, 2018).
Two reviews, Waldron et al. and De Oliveira et al., established a reduction in postoperative pain from a single perioperative dose of dexamethasone in heterogeneous surgical cohorts with debated clinical significance (Moore, 2018; De Oliveira Jr. et al., 2011a; Waldron et al., 2013). Additionally, they demonstrated dexamethasone’s opioid-sparing effects but produced conflicting conclusions regarding the dose-response relationship (De Oliveira Jr. et al., 2011a; Waldron et al., 2013). Therefore, the analgesic benefit of glucocorticoids in abdominal surgery remains unclear (Ahn et al., 2011; Holte & Kehlet, 2002). Waldron et al. excluded patients who received intrathecal or epidural local anaesthetics or opioids yet regional anaesthesia plays a key role in opioid-sparing analgesia for major abdominal surgery (Waldron et al., 2013). Furthermore, patients who received multiple doses of dexamethasone were excluded potentially limiting their clinical significance considering the surgical stress response extends beyond the period of surgery.
Given the exclusion criteria in reviews to date, it is unclear if any benefit demonstrated from the use of dexamethasone in heterogenous cohorts can be translated into patients undergoing elective abdominal surgery.
Therefore, the aim of this review is to determine the effect of perioperative dexamethasone on postoperative pain in adults undergoing general anaesthesia for elective abdominal surgery.
Methods
This study was performed according to a prospectively registered protocol (CRD42020176202) and followed guidance from the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (Moher et al., 2009; Liberati et al., 2009; Research NIfH, 2020).
Randomised controlled trials (RCT) of adults, aged 18 or over, who received intravenous perioperative dexamethasone undergoing general anaesthesia alone or in combination with regional anaesthesia with pain as a primary or secondary outcome for elective abdominal surgery were included. Gastrointestinal, gynaecological and urological procedures were included but renal or transplant surgery was excluded. As the intention was to assess the impact of dexamethasone on postoperative pain, minor gynaecological procedures that were not considered to be painful for example diagnostic laparoscopy were excluded (Alexander, 1997). Studies were included if intravenous dexamethasone was given at any time, in any dose, either alone or in combination with other antiemetics with placebo or any combination of antiemetic drugs as the comparator. Other study drugs could be given provided the analgesic effect of dexamethasone could be isolated. The primary outcome of our review was pain scores reported on an 11-point numerical scale (0–10). Secondary outcomes for this study included time to first analgesia, opioid requirements and time to post anaesthesia care unit (PACU) discharge.
Literature search
Electronic databases Medical Analysis and Retrieval System Online (MEDLINE), Exerpta Medica Database (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science were searched, with no language or date restrictions, for RCTs published up to January 28 2021. When available, a standardised search strategy to identify RCTs was used (Lefebvre et al., 2019) and the full search strategy was published (Research NIfH, 2020) (see Additional file 1). Grey literature and trial registers were searched as pre-specified; however, due to the COVID-19 pandemic, the World Health Organisation (WHO) International Clinical Trials Registry Portal (ICTRP) was temporarily closed to external users and not searched as pre-specified (World Health Organisation, 2020). The reference lists of identified studies and relevant systematic reviews were scanned for additional evidence.
Two authors, (CM and SJC), independently screened unblinded citations, assessed full texts for eligibility, extracted data, recorded on a predetermined data extraction form (see Additional file 2) and assessed bias at outcome level using Cochrane guidance (Sterne et al., 2019). When necessary, a third author (CO’D) mediated any disagreements.
When the specific surgical procedure was not stated and attempts to contact the author failed, we excluded minor painless surgical procedures based on the length of surgery, anaesthetic and surgical technique, length of hospital stay and postoperative analgesic requirements. Pain scores were defined as early (≤ 4 h), intermediate (4–24 h) and late (≥ 24 h) and pain scores presented as a range of times were allocated to the group they most closely corresponded, for example, 0 to 6 h was allocated to the early group. When multiple pain scores were presented for a single time interval, the latest pain score was extracted. Pain scores were assumed to be at rest when this was not stated and converted from a 0–100 to a 0–10 scale as required. Opioids were combined to achieve the total postoperative dose and converted to oral morphine equivalents (see Supplementary Table 1, Additional File 3). Time to first analgesia and PACU discharge were collected in minutes. Authors were successfully contacted for unpublished data or study clarification in seven studies (Bataille et al., 2016; Jo et al., 2012; Sanchez-Ledesma et al., 2002; Ko-Iam et al., 2015; Chen et al., 2020; D'Souza et al., 2011; Kirdak et al., 2008). Data was extracted as mean and standard deviation or converted using verified methods (Higgins & Deeks, 2019; Hozo et al., 2005; Luo et al., 2018; Wan et al., 2014). Studies containing multiple groups were combined into those with dexamethasone, irrespective of dose or timing, and those not containing dexamethasone. When the analgesic effect of dexamethasone could not be isolated, a subset of study data was included to exclude confounding analgesia.
Meta-analysis of outcome data using a random-effects model was performed using Review Manager ((RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020) and presented as mean difference (MD) with 95% confidence intervals (CIs). Statistical heterogeneity was assessed using the method proposed by Higgins et al. (I2 test) (Higgins et al., 2003).
Results
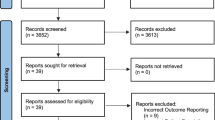
Database and trial registry searches revealed a total of 2160 citations. Altogether, 1846 irrelevant citations were removed, followed by 184 research and publication duplicates leaving 130 articles for eligibility assessment. Twelve articles by Fujii et al. and Schietroma et al. were excluded due to concerns over research validity and multiple retractions (Rasmussen et al., 2012; Carlisle, 2012; Scott, 2012; Myles et al., 2019). We were unable to obtain two full text articles and 13 non-English articles were removed. One hundred and three articles remained for full text eligibility assessment. Studies failed to meet the inclusion criteria and were excluded for the following reasons; 23 articles reported no pain outcomes, three studies were not RCTs, two studies had mixed surgical cohorts, participants did not receive general anaesthesia in three studies, there was no intravenous comparator in four studies and in one the analgesic effect of dexamethasone could not be isolated. Seven studies with minor surgery were excluded (Abreu et al., 2006; Asadollah et al., 2014; Lee et al., 2003; Ormel et al., 2011; Rajeeva et al., 1998; SS, 2007; Thomas & Jones, 2001). A further 12 studies were excluded; two used an alternative method of pain assessment and 10 presented inadequate data for analysis that we were unable to obtain through contacting the authors. Forty-eight full text articles remained, and four additional studies were included after reference list searching resulting in 52 studies with a total of 5758 participants articles (Fig. 1).
The final included studies are summarised in the characteristics of included studies table (Table 1). All 52 studies were RCTs of adult patients undergoing general anaesthesia for abdominal surgery published in English. The most common dose of dexamethasone used was 8 mg but ranged from 1.25 to 20 mg. Four studies presented the dose of dexamethasone in mg kg−1 and were transformed into total doses using the mean study weights or the average weight of an adult at the time and location of the study (De Oliveira Jr. et al., 2011b; Kassim et al., 2018; Lee et al., 2017; Rothenberg et al., 1998; Fryar et al., 2018). No studies administered multiple doses of dexamethasone, but six studies included two or more different doses of dexamethasone (De Oliveira Jr. et al., 2011b; Elhakim et al., 2002; Jokela et al., 2009; Liu et al., 1999; Murphy et al., 2014; Thangaswamy et al., 2010). A further two studies compared the same dose of dexamethasone at different times of administration (Lim et al., 2011; Wang et al., 2000). Opioid doses presented in mg kg−1 were converted in a similar manner to dexamethasone (Ko-Iam et al., 2015; Jokela et al., 2009; Pajunen et al., 2012).
Dexamethasone was directly compared to placebo in 27 studies (Areeruk et al., 2016; Batistaki et al., 2019; Bianchin et al., 2007; Bisgaard et al., 2003; Coloma et al., 2002; Corcoran et al., 2017; De Oliveira Jr. et al., 2011b; Feo et al., 2006; Fukami et al., 2009; Ionescu et al., 2014; Jokela et al., 2009; Lee et al., 2017; Lim et al., 2011; Liu et al., 1998; Liu et al., 1999; Murphy et al., 2011; Murphy et al., 2014; Olajumoke et al., 2013; Pauls et al., 2015; Sanchez-Rodriguez et al., 2010; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015; Wang et al., 1999; Wang et al., 2000; Wu et al., 2009) with a further four comparing dexamethasone to placebo or another antiemetic (Alghanem et al., 2010; Bilgin et al., 2010; Elhakim et al., 2002; Yuksek et al., 2003). Intravenous anti-emetic drugs were included in the intervention or control groups in 17 studies (Bataille et al., 2016; Jo et al., 2012; Sanchez-Ledesma et al., 2002; Ko-Iam et al., 2015; Benevides et al., 2013; Gautam et al., 2008; Hammas et al., 2002; Kasagi et al., 2013; Lopez-Olaondo et al., 1996; Maddali et al., 2003; McKenzie et al., 1997; Nesek-Adam et al., 2007; Pan et al., 2008; Regasa et al., 2020; Rothenberg et al., 1998; Ryu et al., 2013; Shrestha et al., 2014). One study compared dexamethasone with an intraoperative and postoperative propofol infusion (Hammas et al., 2002). Four studies included additional study drugs, but groups were extracted to ensure the analgesic effect of dexamethasone was isolated (Badawy & Sakka, 2015; Kassim et al., 2018; Kurz et al., 2015; Mathiesen et al., 2009).
The timing of dexamethasone varied from 2 h preoperatively to immediately after extubation (Kassim et al., 2018; Thangaswamy et al., 2010; Wang et al., 2000). Dexamethasone was most frequently given preoperatively (Jo et al., 2012; Badawy & Sakka, 2015; Bianchin et al., 2007; Bilgin et al., 2010; Bisgaard et al., 2003; De Oliveira Jr. et al., 2011b; Elhakim et al., 2002; Feo et al., 2006; Fukami et al., 2009; Gautam et al., 2008; Ionescu et al., 2014; Jokela et al., 2009; Kasagi et al., 2013; Kassim et al., 2018; Lee et al., 2017; Lim et al., 2011; Liu et al., 1999; Lopez-Olaondo et al., 1996; Maddali et al., 2003; Mathiesen et al., 2009; Murphy et al., 2011; Pauls et al., 2015; Regasa et al., 2020; Sanchez-Rodriguez et al., 2010; Shrestha et al., 2014; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015; Wang et al., 1999; Wang et al., 2000; Wu et al., 2009; Yuksek et al., 2003), but when administered intraoperatively this was more commonly postinduction pre-incision (Bataille et al., 2016; Sanchez-Ledesma et al., 2002; Alghanem et al., 2010; Areeruk et al., 2016; Batistaki et al., 2019; Benevides et al., 2013; Coloma et al., 2002; Corcoran et al., 2017; Hammas et al., 2002; Kurz et al., 2015; Liu et al., 1998; McKenzie et al., 1997; Murphy et al., 2014; Nesek-Adam et al., 2007; Olajumoke et al., 2013; Pan et al., 2008; Rothenberg et al., 1998; Ryu et al., 2013) than during the surgical procedure (Ko-Iam et al., 2015; Lim et al., 2011). In one study, dexamethasone was given immediately post extubation (Wang et al., 2000).
The primary outcome was most commonly related to postoperative nausea and vomiting (PONV) in 26 studies (Bataille et al., 2016; Jo et al., 2012; Sanchez-Ledesma et al., 2002; Ko-Iam et al., 2015; Alghanem et al., 2010; Benevides et al., 2013; Bianchin et al., 2007; Bilgin et al., 2010; Elhakim et al., 2002; Gautam et al., 2008; Hammas et al., 2002; Kasagi et al., 2013; Liu et al., 1999; Lopez-Olaondo et al., 1996; Maddali et al., 2003; McKenzie et al., 1997; Nesek-Adam et al., 2007; Olajumoke et al., 2013; Pan et al., 2008; Regasa et al., 2020; Rothenberg et al., 1998; Ryu et al., 2013; Wang et al., 1999; Wang et al., 2000; Wu et al., 2009; Yuksek et al., 2003). Pain outcomes were the primary outcome in 11 studies (Areeruk et al., 2016; Badawy & Sakka, 2015; Jokela et al., 2009; Kassim et al., 2018; Lee et al., 2017; Lim et al., 2011; Mathiesen et al., 2009; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015) and was a joint primary outcome in a further six studies (Bisgaard et al., 2003; Feo et al., 2006; Fukami et al., 2009; Liu et al., 1998; Sanchez-Rodriguez et al., 2010; Shrestha et al., 2014). The primary outcome was quality or timing of recovery in four studies (Coloma et al., 2002; De Oliveira Jr. et al., 2011b; Murphy et al., 2011; Pauls et al., 2015), the immune or stress response in two studies (Corcoran et al., 2017; Ionescu et al., 2014), surgical site infection in one study (Kurz et al., 2015), perioperative glucose concentration in one study (Murphy et al., 2014) and reversal of neuromuscular blockade in one study (Batistaki et al., 2019). In general, study outcomes were poorly documented with 25 studies not specifically stating study outcome (Jo et al., 2012; Ko-Iam et al., 2015; Alghanem et al., 2010; Bianchin et al., 2007; Bilgin et al., 2010; Coloma et al., 2002; Corcoran et al., 2017; Elhakim et al., 2002; Gautam et al., 2008; Hammas et al., 2002; Ionescu et al., 2014; Jokela et al., 2009; Lim et al., 2011; Liu et al., 1998; Liu et al., 1999; Nesek-Adam et al., 2007; Olajumoke et al., 2013; Regasa et al., 2020; Rothenberg et al., 1998; Ryu et al., 2013; Shrestha et al., 2014; Sistla et al., 2009; Wang et al., 1999; Wang et al., 2000; Yuksek et al., 2003), seven studies documenting primary outcome only (Sanchez-Ledesma et al., 2002; Badawy & Sakka, 2015; Bisgaard et al., 2003; De Oliveira Jr. et al., 2011b; Maddali et al., 2003; Pan et al., 2008; Wu et al., 2009) and ambiguity over primary or secondary outcomes in a further five studies (Feo et al., 2006; Fukami et al., 2009; Kasagi et al., 2013; Sanchez-Rodriguez et al., 2010; Viriyaroj et al., 2015).
Pain was presented on an 11-point numerical scale in the majority of studies and divided by 10 when presented as 0–100 (Jo et al., 2012; Lee et al., 2017; Mathiesen et al., 2009; Murphy et al., 2011; Murphy et al., 2014; Ryu et al., 2013; Thangaswamy et al., 2010; Tolver et al., 2012). Six studies did not report pain scores (Benevides et al., 2013; Coloma et al., 2002; Hammas et al., 2002; Ionescu et al., 2014; Olajumoke et al., 2013; Regasa et al., 2020) and we were unable to extract pain scores in a further four studies (Bisgaard et al., 2003; Kassim et al., 2018; Liu et al., 1998; Lopez-Olaondo et al., 1996). The pain outcomes extracted from each study are presented in Table 2.
Bias assessment judged seven studies to be low risk (Sanchez-Ledesma et al., 2002; Gautam et al., 2008; Kassim et al., 2018; McKenzie et al., 1997; Murphy et al., 2011; Thangaswamy et al., 2010; Tolver et al., 2012), 20 studies to have some concerns (Bataille et al., 2016; Jo et al., 2012; Alghanem et al., 2010; Badawy & Sakka, 2015; Benevides et al., 2013; Coloma et al., 2002; Feo et al., 2006; Fukami et al., 2009; Kasagi et al., 2013; Lee et al., 2017; Lim et al., 2011; Lopez-Olaondo et al., 1996; Murphy et al., 2014; Nesek-Adam et al., 2007; Olajumoke et al., 2013; Pan et al., 2008; Rothenberg et al., 1998; Sanchez-Rodriguez et al., 2010; Shrestha et al., 2014; Viriyaroj et al., 2015) and 25 to be high risk (Ko-Iam et al., 2015; Areeruk et al., 2016; Batistaki et al., 2019; Bianchin et al., 2007; Bilgin et al., 2010; Bisgaard et al., 2003; Corcoran et al., 2017; De Oliveira Jr. et al., 2011b; Elhakim et al., 2002; Hammas et al., 2002; Ionescu et al., 2014; Jokela et al., 2009; Kurz et al., 2015; Liu et al., 1998; Liu et al., 1999; Maddali et al., 2003; Mathiesen et al., 2009; Pauls et al., 2015; Regasa et al., 2020; Ryu et al., 2013; Sistla et al., 2009; Wang et al., 1999; Wang et al., 2000; Wu et al., 2009; Yuksek et al., 2003). For risk of bias (ROB) assessment, see Supplementary Table 2, Additional File 4 and Supplementary Fig. 1 and 2, Additional File 5.
Pain scores
Early pain scores at rest were recorded in 30 studies (3408 patients) (Bataille et al., 2016; Jo et al., 2012; Sanchez-Ledesma et al., 2002; Alghanem et al., 2010; Badawy & Sakka, 2015; Batistaki et al., 2019; Bianchin et al., 2007; Corcoran et al., 2017; De Oliveira Jr. et al., 2011b; Feo et al., 2006; Fukami et al., 2009; Gautam et al., 2008; Jokela et al., 2009; Kasagi et al., 2013; Kurz et al., 2015; Lim et al., 2011; Mathiesen et al., 2009; McKenzie et al., 1997; Murphy et al., 2011; Murphy et al., 2014; Nesek-Adam et al., 2007; Pan et al., 2008; Ryu et al., 2013; Sanchez-Rodriguez et al., 2010; Sistla et al., 2009; Thangaswamy et al., 2010; Viriyaroj et al., 2015; Wang et al., 1999; Wang et al., 2000; Wu et al., 2009) with a statistically significant reduction in pain in patients receiving dexamethasone (MD − 0.54; CI − 0.72, − 0.35; I2 81%; n = 3408) (Fig. 2). The direction of result remained unchanged when the analysis was restricted to studies with pain (MD − 0.8; CI − 1.22, − 0.38; I2 91%; n = 950) and non-pain (MD − 0.4; CI − 0.62, − 0.19; I2 63%; n = 2458) primary outcomes.
Ten studies (1319 patients) reported early pain scores on movement (Bataille et al., 2016; Corcoran et al., 2017; Jokela et al., 2009; Lee et al., 2017; Mathiesen et al., 2009; Murphy et al., 2011; Murphy et al., 2014; Nesek-Adam et al., 2007; Sistla et al., 2009; Thangaswamy et al., 2010) with a statistically significant reduction in pain in patients who received dexamethasone (MD − 0.42; CI − 0.62, − 0.22; I2 35%; n = 1319). The result trend did not vary when the analysis was limited to studies with non-pain (MD − 0.47; CI − 0.84, − 0.10; I2 52%; n = 618) or pain (MD − 0.43; CI − 0.68, − 0.18; I2 17%; n = 701) as the primary outcome.
Intermediate pain scores at rest were recorded in 27 studies (3022 patients) (Jo et al., 2012; Sanchez-Ledesma et al., 2002; Ko-Iam et al., 2015; Areeruk et al., 2016; Badawy & Sakka, 2015; Batistaki et al., 2019; Bianchin et al., 2007; Bilgin et al., 2010; Feo et al., 2006; Fukami et al., 2009; Gautam et al., 2008; Jokela et al., 2009; Kasagi et al., 2013; Kurz et al., 2015; Lim et al., 2011; Maddali et al., 2003; Murphy et al., 2011; Nesek-Adam et al., 2007; Pan et al., 2008; Rothenberg et al., 1998; Ryu et al., 2013; Sanchez-Rodriguez et al., 2010; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015; Yuksek et al., 2003) and on movement in nine studies (1112 patients) (Sanchez-Ledesma et al., 2002; Areeruk et al., 2016; Jokela et al., 2009; Lee et al., 2017; Murphy et al., 2011; Nesek-Adam et al., 2007; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012). There was a statistically significant reduction in intermediate pain scores both at rest (MD − 0.31; CI − 0.47, − 0.14; I2 96%; n = 3022) and on movement (MD − 0.26; CI − 0.39, − 0.13; I2 29%; n = 1112) in patients receiving dexamethasone. When analysis of intermediate pain scores at rest was restricted to studies with pain as the primary outcome, the direction of result remained (MD − 0.57; CI − 0.92, − 0.22; I2 89%; n = 996); however, lost statistical significance when restricted to non-pain primary outcomes (MD − 0.18; CI − 0.39, 0.03; I2 97%; n = 2026). Restricting the results for intermediate pain scores on movement to pain (MD − 0.33; CI − 0.45, − 0.21; I2 0%; n = 747) and non-pain (MD − 0.16; CI − 0.25, − 0.07; I2 0%; n = 365) primary outcomes did not change the direction of the result.
Late pain scores at rest were recorded in 25 studies (2443 patients) (Bataille et al., 2016; Sanchez-Ledesma et al., 2002; Alghanem et al., 2010; Areeruk et al., 2016; Badawy & Sakka, 2015; Batistaki et al., 2019; Bianchin et al., 2007; Elhakim et al., 2002; Feo et al., 2006; Fukami et al., 2009; Gautam et al., 2008; Jokela et al., 2009; Lim et al., 2011; Liu et al., 1999; Mathiesen et al., 2009; McKenzie et al., 1997; Pan et al., 2008; Pauls et al., 2015; Ryu et al., 2013; Sanchez-Rodriguez et al., 2010; Shrestha et al., 2014; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015). There was a statistically significant reduction in pain scores in patients who received dexamethasone (MD − 0.38; CI − 0.52, − 0.24; I2 88%; n = 2443). The direction of the result was unchanged when the study outcome was restricted to pain (MD − 0.42; CI − 0.68, − 0.16; I2 90%; n = 1192) and non-pain (MD − 0.34; CI − 0.57, − 0.11; I2 77%; n = 1251) primary outcomes.
Ten studies (1210 patients) (Bataille et al., 2016; Sanchez-Ledesma et al., 2002; Areeruk et al., 2016; Elhakim et al., 2002; Jokela et al., 2009; Lee et al., 2017; Mathiesen et al., 2009; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012) reported late pain on movement with a statistically significant reduction in pain scores in patients who received dexamethasone (MD − 0.38; CI − 0.65, − 0.11; I2 71%; n = 1210). Confining the results to non-pain primary outcomes did not change the result trend (MD − 0.49; CI − 0.95, − 0.03; I2 59%; n = 387) but limiting to studies with pain as the primary outcome demonstrated no statistical significance (MD − 0.3; CI − 0.61, 0.00; I2 66%; n = 823).
Analgesic requirements
Time to first analgesia was recorded in 12 studies (1581 patients) (De Oliveira et al., 2011; Kassim et al., 2018; Lee et al., 2017; Elhakim et al., 2002; Jokela et al., 2009; Liu et al., 1999; Thangaswamy et al., 2010; Coloma et al., 2002; Yuksek et al., 2003; Gautam et al., 2008; Nesek-Adam et al., 2007; Badawy & Sakka, 2015) Listed as numbers and not the same as above. There was a statistically significant increase in time to first analgesia (minutes) in patients who received dexamethasone (MD 22.92.; CI 11.09, 34.75; I2 99%; n = 1581) (Fig. 3). Restricting the analysis to studies with pain (MD 31.97; CI 13.35, 50.60; I2 99%; n = 643) and non-pain primary outcomes (MD 15.17; CI 0.33, 30.02; I2 91%; n = 938) did not affect the trend.
Postoperative opioids were recorded in 33 studies (3339 patients) (Sanchez-Ledesma et al., 2002; Ko-Iam et al., 2015; Areeruk et al., 2016; Badawy & Sakka, 2015; Benevides et al., 2013; Bisgaard et al., 2003; Coloma et al., 2002; De Oliveira Jr. et al., 2011b; Elhakim et al., 2002; Gautam et al., 2008; Hammas et al., 2002; Ionescu et al., 2014; Jokela et al., 2009; Kassim et al., 2018; Lee et al., 2017; Lim et al., 2011; Liu et al., 1998; Liu et al., 1999; Lopez-Olaondo et al., 1996; Mathiesen et al., 2009; McKenzie et al., 1997; Murphy et al., 2011; Murphy et al., 2014; Pan et al., 2008; Pauls et al., 2015; Regasa et al., 2020; Rothenberg et al., 1998; Sistla et al., 2009; Thangaswamy et al., 2010; Tolver et al., 2012; Viriyaroj et al., 2015; Wang et al., 1999; Wang et al., 2000). However, there was variability in the type, administration and time of recorded opioids varying from one hour to five days postoperatively. There was a statistically significant reduction in opioid use (mg of oral morphine equivalents) in patients who received dexamethasone (MD − 6.66; CI − 9.38, − 3.93; I2 88%; n = 3339) (Fig. 4). Statistical significance remained when the result was restricted to pain (MD − 8.35; CI − 11.64, − 5.07; I2 58%; n = 1251) and non-pain (MD − 5.50; CI − 9.15, − 1.85; I2 91%; n = 2088) primary outcomes. Visual inspection of the funnel plots for total opioid requirements and early pain scores at rest do not suggest evidence of significant reporting or publication bias (see Supplementary Fig. 3 and Fig. 4, Additional File 6).
Time to PACU discharge
Nine studies (947 patients) reported time to discharge from PACU (Benevides et al., 2013; Bisgaard et al., 2003; Coloma et al., 2002; De Oliveira Jr. et al., 2011b; Murphy et al., 2011; Murphy et al., 2014; Olajumoke et al., 2013; Pan et al., 2008; Rothenberg et al., 1998). There was no difference in time to PACU discharge between patients who received dexamethasone and those who did not (MD − 3.82; CI − 10.87, 3.23; I2 59%; n = 947). Removing the single study with pain as the primary outcome and restricting the analysis to non-pain (MD − 4.37; CI − 12.10, 3.37; I2 54%; n = 867) had no impact on the result.
Subgroup analyses
Subgroup analyses of general anaesthesia in combination with either central neuraxial blockade (GA + CNB) or regional anaesthesia (GA + RA) were previously documented (CRD42020176202) (Research NIfH, 2020). Patients received GA + CNB in three studies; spinal with intrathecal morphine (Sanchez-Ledesma et al., 2002), epidural administration of morphine and fentanyl (Yuksek et al., 2003) and a small proportion of both the intervention and control groups received an epidural in one study (Kurz et al., 2015). The subset of study data was not available in this study (Kurz et al., 2015). One study documented the use of regional anaesthesia with either transversus abdominal plane block or rectus sheath block (Regasa et al., 2020). Given the limited data, these predefined subgroup analyses were not undertaken.
The planned dosing subgroup analyses were undertaken for a single but not multiple doses of dexamethasone. Doses were grouped pragmatically into three categories to correspond with clinical practice; low dose 1.25–5 mg, intermediate dose 6.4–10 mg and high dose 11–20 mg. For early pain scores at rest, both low (MD − 0.55; CI − 1.04, − 0.07; I2 66%; n = 1023) and intermediate (MD − 0.55; CI − 0.76, − 0.34; I2 83%; n = 2265) demonstrated benefit with no impact from high dose (MD − 0.21; CI − 1.02, 0.60; I2 0%; n = 120). For early pain scores on movement, only intermediate dose (MD − 0.48; CI − 0.75, − 0.21; I2 47; n = 587) demonstrated benefit with no impact from low (MD − 0.34; CI − 0.67, 0.00; I2 0%; n = 692) or high dose (MD − 0.40; CI − 1.79, 0.99; n = 40).
For 4–24-h pain scores, again, there was evidence of dose response for intermediate dose at rest (MD − 0.36; CI − 0.53, − 0.18; I2 96%; n = 2221) and on movement (MD − 0.25; CI − 0.37, − 0.13; I2 22%, n = 1005). There was a lack of statistical significance for low (MD 0.22; CI − 0.15, 0.58; I2 0%, n = 666) and high dose (MD − 0.05; CI − 0.76, 0.66; I2 0%; n = 135) at rest and low (MD − 0.12; CI − 0.77, 0.54; I2 0%; n = 67) and high dose (MD − 0.70; CI − 2.62, 1.22; n = 40) on movement.
Intermediate dose remained statistically significant (MD − 0.42; CI − 0.62, − 0.22; I2 84%; n = 1847) for late pain scores at rest but low (MD − 0.08; CI − 0.22, 0.06; I2 19%; n = 431) and high (MD − 0.51; CI − 1.32, 0.30; I2 66%; n = 165) dose dexamethasone demonstrated no difference (Fig. 5). This pattern was mirrored in late pain scores on movement; low (MD − 0.25; CI − 0.5, 0.00; I2 0%; n = 274), intermediate (MD − 0.47; CI − 0.83, − 0.10; I2 70%; n = 851) and high dose (MD − 0.31; CI − 1.43, 0.82; I2 74%; n = 85).
Time to first analgesia was increased with intermediate dose (MD 27.76; CI 13.96, 41.55; I2 98%; n = 1034) but low (MD 11.58; CI − 0.34, 23.5; I2 89%; n = 462) and high dose had no impact (MD 25.44; CI − 2.23, 53.12; I2 86%; n = 85). Again, a statistically significant reduction in postoperative opioid requirements was maintained for intermediate dose (MD − 7.20; CI − 9.77, − 4.64; I2 80%; n = 2402) but low (MD − 8.14; CI − 16.72, 0.44; I2 89%; n = 677) and high dose dexamethasone (MD − 19.26; CI − 57.79, 19.28; I2 94%; n = 260) demonstrated no difference.
Subgroup analysis did not impact time to PACU discharge with no difference from low (MD 0.27; CI − 6.72, 7.27; I2 40%; n = 385), intermediate (MD − 9.56; CI − 24.56, 5.44; I2 65%; n = 467) or high (MD − 3.76; CI − 15.77, 8.25; n = 95) dose dexamethasone (see supporting information, Appendix 3).
Timing of administration subgroup analyses of dexamethasone were also performed. This was categorised as preoperative (before anaesthetic induction), intraoperative (anaesthetic induction and to extubation) and postoperative (after extubation). The timing subgroup analyses demonstrated a global reduction in pain scores from preoperative administration of dexamethasone for all pain scores both at rest and on movement. In contrast, intraoperative administration only reduced late pain scores at rest.
Preoperative dexamethasone significantly increased time to first analgesia (MD 28.13; CI 14.57, 41.68; I2 98%; n = 1281), but there was no difference from intraoperative administration (MD − 0.01; CI − 6.24, 6.21; I2 0%; n = 300). Additionally, preoperative dexamethasone decreased total opioid administration (MD − 8.55; CI − 12.34, − 4.76; I2 89%; n = 2214) with no effect from intraoperative administration (MD − 2.18; CI − 5.93, 1.56; I2 83%; n = 1065). Postoperative dexamethasone (MD − 12.00; CI − 17.45, − 6.55; n = 40) decreased opioid administration but this was based on results from a single study (Wang et al., 2000). Time to PACU discharge remained unaffected by dexamethasone timing; preoperative (MD − 12.55; CI − 30.73, 5.63; I2 62%; n = 361), intraoperative (MD − 0.56; CI − 7.41, 6.29; I2 57%; n = 586) (For additional forest plots see Additional File 7).
Conclusions
To our knowledge, this is the largest systematic review and meta-analysis investigating the effect of perioperative dexamethasone on postoperative pain in adults undergoing elective abdominal surgery under general anaesthesia and the first to demonstrate an important analgesic effect in this surgical cohort.
Our analyses demonstrated a statistically significant reduction in early, intermediate and late pain scores both at rest and on movement. Sub-group analyses revealed that intermediate dose (6.4–10 mg) effectively decreased pain at all time intervals both at rest and on movement. However, low dose (1.25–5 mg) only affected early pain scores at rest while high dose (11–20 mg) had no impact on any pain scores. Preoperative administration of dexamethasone demonstrated a global reduction on all pain scores. Intraoperative administration was more beneficial in reducing late pain scores at rest but failed to impact pain at any other time period. Dexamethasone also reduced the total postoperative opioid requirements and increased the time to first analgesia with intermediate dose (6.4–10 mg) and preoperative administration demonstrating the greatest impact. Time to PACU discharge was not altered by dexamethasone at any dose or time and is likely to be influenced by external factors (Samad et al., 2006). However, this is contrary to previous findings which have questionable clinical significance (Waldron et al., 2013).
Dexamethasone’s established anti-inflammatory properties have ensured it is a widely used effective perioperative anti-emetic (Moore, 2018; Holte & Kehlet, 2002; De Oliveira Jr. et al., 2013). In abdominal surgery, glucocorticoids reduce pro-inflammatory mediators and phospholipase required for pain pathways allowing its analgesic benefits to be increasingly recognized (Moore, 2018; De Oliveira Jr. et al., 2011a; Waldron et al., 2013; Holte & Kehlet, 2002). Enhanced recovery pathways encouraging earlier mobility have boosted the demand for opioid-sparing multimodal analgesia in patients undergoing abdominal surgery (Gustafsson et al., 2019; Nelson et al., 2019; Nygren et al., 2012; Thorell et al., 2016). Dexamethasone has, therefore, an important role in postoperative analgesia with additional benefit for multimodal analgesic regimes in this patient population. However, full analgesic effect is unlikely from the commonly used lower anti-emetic dose and intermediate dose (6.4–10 mg) is necessary to produce global reductions in pain scores, increase time to first analgesia and reduce opioid requirements (Moore, 2018; De Oliveira Jr. et al., 2013). Additionally, timing of administration is crucial as the analgesic benefits of preoperative dexamethasone far outweigh administration at induction as recommended for antiemetic effect (Gan et al., 2020).
One of the major strengths of this review is inclusion of a large number of studies and participants of a relatively homogenous surgical population. This allows the results to inform future clinical practice and guidelines in moderate and major abdominal surgery. A previous systematic review failed to demonstrate a reduction in early pain scores on movement from dexamethasone administration, likely due to small numbers (Waldron et al., 2013). This new finding is potentially significant for enhanced recovery regimes where early movement after abdominal surgery is encouraged (Gustafsson et al., 2019; Nelson et al., 2019; Nygren et al., 2012; Thorell et al., 2016). In addition, investigation of dexamethasone’s effect on intermediate pain scores is novel and provides further evidence of its analgesic effects (De Oliveira Jr. et al., 2011a; Waldron et al., 2013). Through subgroup analyses, we have provided clarification on the debated perioperative dosing and given strength to the previously suggested preoperative timing (De Oliveira Jr. et al., 2011a; Waldron et al., 2013). Despite demonstrating a globally statistically significant reduction in postoperative pain scores, it is important to remember that the clinical significance of this is uncertain. The increase in time to first analgesia and reduction in postoperative opioids is likely to have more clinical impact on patients undergoing abdominal surgery. When studies with regional anaesthesia were removed, a statistically significant reduction in postoperative opioids (MD − 6.87; CI − 9.70, − 4.05; I2 89%; n = 3153) and increased time to first analgesia (MD 23.01; CI 11.14, 34.88; I2 98%; n = 1521) remained.
There are a number of limitations in our review. Firstly, results could potentially be biased by selective reporting and missing outcome data, but the funnel plots were reassuring (Chen et al., 2020; D'Souza et al., 2011; Kirdak et al., 2008; Bala et al., 2014; Bilgin et al., 2004; Chu et al., 2008; Karaman et al., 2013; McKenzie et al., 1994; Ramesh, 2011; Wang et al., 2002; Zargar-Shoshtari et al., 2009). Secondly, as the latest pain score was extracted from each time interval, there could be significant variation in the timing which may explain some of the statistical heterogenicity in intermediate and late pain scores. Late pain scores varied from 24 h to 4 days, with later pain scores less likely to demonstrate statistical significance potentially influencing the results. The variation in timing of recorded postoperative opioid consumption, from 1 h to 5 days, may also account for some of the statistical heterogenicity. Thirdly, results from the high dose and postoperative subgroup analyses should be interpreted with caution given the low numbers available. In addition, we did not investigate the impact of adverse effects of dexamethasone administration as this has previously been done (De Oliveira Jr. et al., 2011a; Waldron et al., 2013; Polderman et al., 2018). However, when reported, adverse features reported were similar between intervention and control groups and not attributed to dexamethasone administration.
Furthermore, pain was the primary outcome in less than half the studies but when analyses were restricted to studies with pain as the primary outcome all results remained statistically significant except late pain scores on movement. In addition, pain scores on movement were less likely to be reported potentially reducing the strength of the sensitivity analyses. Pain scores on movement should be the focus of future studies given the drive for postoperative mobilisation.
Additionally, due to lack of data, we were unable to perform our prespecified subgroup analyses GA + CNB and GA + RA. Dexamethasone may impact on postoperative pain in combination with general and regional anaesthesia, but it is unclear if this can be translated to the general surgical population (Chen et al., 2018; Fan et al., 2018; Pehora et al., 2017). It is our opinion that this question remains unanswered and should guide future research.
Unfortunately, nearly half of all studies were deemed high ROB, frequently due to selection of the reported result with failure to report all measured pain scores. As the majority of studies had a non-pain primary outcome, ROB assessment at study rather than outcome level would have impacted these results. ROB assessment highlighted issues with study methodology, with inadequate allocation concealment in nearly half of all studies, and trialists should be reminded of reporting guidelines for RCTs (Moher et al., 2010). Additionally, the type of analysis was infrequently documented, and we judged nearly half of all studies undertook a per-protocol analysis due to exclusions of protocol violations and post-randomisation participants for reasons not pre-specified. Some exclusions are justified in a modified intention-to-treat (mITT) analysis, but we exercised caution using this label due to ambiguity over the definition (Abraha & Montedori, 2010; Gupta, 2011). We feel clarification of mITT criteria is essential to avoid subjectivity of future ROB assessments. However, the completeness of outcome data provides some reassurance over the safety and lack of adverse features of dexamethasone.
In conclusion, a single perioperative dose of intravenous dexamethasone reduces early, intermediate and late pain scores both at rest and on movement, opioid requirements and increases time to first analgesia in patients undergoing elective abdominal surgery. Preoperative administration of intermediate dose is likely to have the greatest impact on outcomes.
Availability of data and materials
All data collected and analysed for the current study are available from the corresponding author on reasonable request.
Abbreviations
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- CNB:
-
Central neuraxial blockade
- EMBASE:
-
Exerpta Medica Database
- GA:
-
General anaesthesia
- ICTRP:
-
International Clinical Trials Registry Portal
- MEDLINE:
-
Medical Analysis and Retrieval System Online
- MD:
-
Mean difference
- mITT:
-
Modified intention to treat
- PACU:
-
Post anaesthesia care unit
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- RA:
-
Regional anaesthesia
- RevMan:
-
Review manager
- RCT:
-
Randomised controlled trial
- ROB:
-
Risk of bias
- PONV:
-
Postoperative nausea and vomiting
- WHO:
-
World Health Organisation
References
Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ. 2010;340(jun14 1):c2697. https://doi.org/10.1136/bmj.c2697.
Abreu MP, Vieira JL, Silva IF, Miziara LE, Fofano R. Efficacy of ondansetron, metoclopramide, droperidol and dexametasone in preventing post-gynecological videolaparoscopy nausea and vomiting in outpatient setting. Comparative study. Rev Bras Anestesiol. 2006;56(1):8–15.
Ahn Y, Woods J, Connor S. A systematic review of interventions to facilitate ambulatory laparoscopic cholecystectomy. HPB (Oxford). 2011;13(10):677–86. https://doi.org/10.1111/j.1477-2574.2011.00371.x.
Alexander JI. Pain after laparoscopy. Br J Anaesth. 1997;79(3):369–78. https://doi.org/10.1093/bja/79.3.369.
Alghanem SM, Massad IM, Rashed EM, Abu-Ali HM, Daradkeh SS. Optimization of anesthesia antiemetic measures versus combination therapy using dexamethasone or ondansetron for the prevention of postoperative nausea and vomiting. Surg Endosc. 2010;24(2):353–8. https://doi.org/10.1007/s00464-009-0567-3.
Areeruk P, Ittichaikulthol W, Termpornlert S, Pravitharangul T, Nganlasom J, Charoensap C, et al. The effect of a single dose dexamethasone on postoperative pain in patients undergoing gynecological laparotomy surgery. J Med Assoc Thai. 2016;99(11):1239–44.
Asadollah S, Vahdat M, Yazdkhasti P, Nikravan N. The influence of dexamethasone on postoperative nausea and vomiting in patients undergoing gynecologic laparoscopic surgeries: a randomised, controlled, double blind trial. Turk J Obstet Gynecol. 2014;11(4):219–23. https://doi.org/10.4274/tjod.13471.
Badawy AA, Sakka AE. Preoperative gabapentin alone or in combination with dexamethasone on postoperative pain relief after abdominal hysterectomies. A randomized controlled trial. Egyptian Journal of Anaesthesia. 2015;31(2):107–13. https://doi.org/10.1016/j.egja.2014.12.010.
Bala I, Bharti N, Murugesan S, Gupta R. Comparison of palonosetron with palonosetron-dexamethasone combination for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2014;80(7):779–84.
Bataille A, Letourneulx JF, Charmeau A, Lemedioni P, Leger P, Chazot T, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anaesthesia: a randomised double-blind placebo-controlled study. Eur J Anaesthesiol. 2016;33(12):898–905. https://doi.org/10.1097/EJA.0000000000000427.
Batistaki C, Pandazi A, Kyttari A, Kaminiotis E, Kostopanagiotou G. Is there an interaction between dexamethasone and sugammadex in real clinical conditions? A randomized controlled trial in patients undergoing laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2019;35(2):215–9. https://doi.org/10.4103/joacp.JOACP_42_17.
Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23(9):1389–96. https://doi.org/10.1007/s11695-013-0923-1.
Bianchin A, De Luca A, Caminiti A. Postoperative vomiting reduction after laparoscopic cholecystectomy with single dose of dexamethasone. Minerva Anestesiol. 2007;73(6):343–6.
Bilgin TE, Birbicer H, Doruk N, Altunkan A, Camdeviren H, Akca O, et al. The role of dexamethasone on postoperative nausea and vomiting after gynaecological surgery: a double-blind placebo controlled comparative study with ondansetron and metoclopramide.: European Journal of Anaesthesiology; 2004. p. 18.
Bilgin TE, Birbicer H, Ozer Z, Doruk N, Tok E, Oral U. A comparative study of the antiemetic efficacy of dexamethasone, ondansetron, and metoclopramide in patients undergoing gynecological surgery. Med Sci Monit. 2010;16(7):CR336–41.
Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg. 2003;238(5):651–60. https://doi.org/10.1097/01.sla.0000094390.82352.cb.
Carlisle JB. The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia. 2012;67(5):521–37. https://doi.org/10.1111/j.1365-2044.2012.07128.x.
Chen Q, An R, Zhou J, Yang B. Clinical analgesic efficacy of dexamethasone as a local anesthetic adjuvant for transversus abdominis plane (TAP) block: a meta-analysis. PLoS One. 2018;13(6):e0198923. https://doi.org/10.1371/journal.pone.0198923.
Chen Y, Dong C, Lian G, Li D, Yin Y, Yu W, et al. Dexamethasone on postoperative gastrointestinal motility: A placebo-controlled, double-blinded, randomized controlled trial. J Gastroenterol Hepatol. 2020;35(9):1549–54. https://doi.org/10.1111/jgh.15020.
Chu CC, Shieh JP, Tzeng JI, Chen JY, Lee Y, Ho ST, et al. The prophylactic effect of haloperidol plus dexamethasone on postoperative nausea and vomiting in patients undergoing laparoscopically assisted vaginal hysterectomy. Anesth Analg. 2008;106(5):1402-1406, table of contents.
Coloma M, White PF, Markowitz SD, Whitten CW, Macaluso AR, Berrisford SB, et al. Dexamethasone in combination with dolasetron for prophylaxis in the ambulatory setting: effect on outcome after laparoscopic cholecystectomy. Anesthesiology. 2002;96(6):1346–50. https://doi.org/10.1097/00000542-200206000-00013.
Corcoran T, Paech M, Law D, Muchatuta NA, French M, Ho KM. Intraoperative dexamethasone alters immune cell populations in patients undergoing elective laparoscopic gynaecological surgery. Br J Anaesth. 2017;119(2):221–30. https://doi.org/10.1093/bja/aex154.
De Oliveira GS Jr, Ahmad S, Fitzgerald PC, Marcus RJ, Altman CS, Panjwani AS, et al. Dose ranging study on the effect of preoperative dexamethasone on postoperative quality of recovery and opioid consumption after ambulatory gynaecological surgery. Br J Anaesth. 2011b;107(3):362–71. https://doi.org/10.1093/bja/aer156.
De Oliveira GS Jr, Almeida MD, Benzon HT, RJ MC. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011a;115(3):575–88. https://doi.org/10.1097/ALN.0b013e31822a24c2.
De Oliveira GS Jr, Castro-Alves LJ, Ahmad S, Kendall MC, RJ MC. Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg. 2013;116(1):58–74. https://doi.org/10.1213/ANE.0b013e31826f0a0a.
D'Souza N, Swami M, Bhagwat S. Comparative study of dexamethasone and ondansetron for prophylaxis of postoperative nausea and vomiting in laparoscopic gynecologic surgery. Int J Gynaecol Obstet. 2011;113(2):124–7. https://doi.org/10.1016/j.ijgo.2010.11.022.
Elhakim M, Nafie M, Mahmoud K, Atef A. Dexamethasone 8 mg in combination with ondansetron 4 mg appears to be the optimal dose for the prevention of nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth. 2002;49(9):922–6. https://doi.org/10.1007/BF03016875.
Fan Z, Ma J, Kuang M, Zhang L, Han B, Yang B, et al. The efficacy of dexamethasone reducing postoperative pain and emesis after total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2018;52:149–55. https://doi.org/10.1016/j.ijsu.2018.02.043.
Feo CV, Sortini D, Ragazzi R, De Palma M, Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93(3):295–9. https://doi.org/10.1002/bjs.5252.
Fryar CDK-MD, Gu Q, Ogden CL. Mean body weight, height, waist circumference, and body mass index among adults: United States 1999-2000 through 2015-2016. Natl Health Stat Rep. 2018;122:1–16.
Fukami Y, Terasaki M, Okamoto Y, Sakaguchi K, Murata T, Ohkubo M, et al. Efficacy of preoperative dexamethasone in patients with laparoscopic cholecystectomy: a prospective randomized double-blind study. J Hepatobiliary Pancreat Surg. 2009;16(3):367–71. https://doi.org/10.1007/s00534-009-0079-5.
Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–48. https://doi.org/10.1213/ANE.0000000000004833.
Gautam B, Shrestha BR, Lama P, Rai S. Antiemetic prophylaxis against postoperative nausea and vomiting with ondansetron-dexamethasone combination compared to ondansetron or dexamethasone alone for patients undergoing laparoscopic cholecystectomy. Kathmandu Univ Med J (KUMJ). 2008;6(23):319–28.
Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–12. https://doi.org/10.4103/2229-3485.83221.
Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS((R))) Society Recommendations: 2018. World J Surg. 2019;43(3):659–95. https://doi.org/10.1007/s00268-018-4844-y.
Hammas B, Thorn SE, Wattwil M. Superior prolonged antiemetic prophylaxis with a four-drug multimodal regimen—comparison with propofol or placebo. Acta Anaesthesiol Scand. 2002;46(3):232–7. https://doi.org/10.1034/j.1399-6576.2002.460302.x.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195(5):694–712. https://doi.org/10.1016/S1072-7515(02)01491-6.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. https://doi.org/10.1186/1471-2288-5-13.
Ionescu DC, Hadade AI, Mocan TA, Margarit SD. The influence of a prophylactic dose of dexamethasone for postoperative nausea and vomiting on plasma interleukin concentrations after laparoscopic cholecystectomy: a randomised trial. Eur J Anaesthesiol. 2014;31(4):204–11. https://doi.org/10.1097/EJA.0b013e3283642a01.
Jo YY, Lee JW, Shim JK, Lee WK, Choi YS. Ramosetron, dexamethasone, and their combination for the prevention of postoperative nausea and vomiting in women undergoing laparoscopic cholecystectomy. Surg Endosc. 2012;26(8):2306–11. https://doi.org/10.1007/s00464-012-2180-0.
Jokela RM, Ahonen JV, Tallgren MK, Marjakangas PC, Korttila KT. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–15. https://doi.org/10.1213/ane.0b013e3181ac0f5c.
Karaman K, Bostanci EB, Aksoy E, Ulas M, Yigit T, Erdemli MO, et al. Effects of dexamethasone and pheniramine hydrogen maleate on stress response in patients undergoing elective laparoscopic cholecystectomy. Am J Surg. 2013;205(2):213–9. https://doi.org/10.1016/j.amjsurg.2012.05.010.
Kasagi Y, Hayashida M, Sugasawa Y, Kikuchi I, Yamaguchi K, Okutani R, et al. Antiemetic effect of naloxone in combination with dexamethasone and droperidol in patients undergoing laparoscopic gynecological surgery. J Anesth. 2013;27(6):879–84. https://doi.org/10.1007/s00540-013-1630-8.
Kassim D, Esmat I, Elgendy M. Impact of duloxetine and dexamethasone for improving postoperative pain after laparoscopic gynecological surgeries: a randomized clinical trial. Saudi J Anaesth. 2018;12(1):95–102. https://doi.org/10.4103/sja.SJA_519_17.
Kirdak T, Yilmazlar A, Cavun S, Ercan I, Yilmazlar T. Does single, low-dose preoperative dexamethasone improve outcomes after colorectal surgery based on an enhanced recovery protocol? Double-blind, randomized clinical trial. Am Surg. 2008;74(2):160–7. https://doi.org/10.1177/000313480807400216.
Ko-Iam W, Sandhu T, Paiboonworachat S, Pongchairerks P, Junrungsee S, Chotirosniramit A, et al. Metoclopramide, versus its combination with dexamethasone in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: a double-blind randomized controlled trial. J Med Assoc Thai. 2015;98(3):265–72.
Kurz A, Fleischmann E, Sessler DI, Buggy DJ, Apfel C, Akca O, et al. Effects of supplemental oxygen and dexamethasone on surgical site infection: a factorial randomized trialdouble dagger. Br J Anaesth. 2015;115(3):434–43. https://doi.org/10.1093/bja/aev062.
Lee C, Chung JY, Lee M. Sex-related differences in the efficacy of dexamethasone pretreatment for postoperative analgesia in patients undergoing laparoscopic cholecystectomy: a randomized controlled study. Turk J Med Sci. 2017;47(4):1282–6. https://doi.org/10.3906/sag-1701-113.
Lee Y, Lai HY, Lin PC, Huang SJ, Lin YS. Dexamethasone prevents postoperative nausea and vomiting more effectively in women with motion sickness. Can J Anaesth. 2003;50(3):232–7. https://doi.org/10.1007/BF03017790.
Lefebvre C GJ, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I et al. Technical supplement to chapter 4: searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. 2019. In: Cochrane [Internet]. [94 pages]. Available from: www.training.cochrane.org/handbook.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. https://doi.org/10.1136/bmj.b2700.
Lim SH, Jang EH, Kim MH, Cho K, Lee JH, Lee KM, et al. Analgesic effect of preoperative versus intraoperative dexamethasone after laparoscopic cholecystectomy with multimodal analgesia. Korean J Anesthesiol. 2011;61(4):315–9. https://doi.org/10.4097/kjae.2011.61.4.315.
Liu K, Hsu CC, Chia YY. Effect of dexamethasone on postoperative emesis and pain. Br J Anaesth. 1998;80(1):85–6. https://doi.org/10.1093/bja/80.1.85.
Liu K, Hsu CC, Chia YY. The effective dose of dexamethasone for antiemesis after major gynecological surgery. Anesth Analg. 1999;89(5):1316–8. https://doi.org/10.1213/00000539-199911000-00046.
Lopez-Olaondo L, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Saez A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76(6):835–40. https://doi.org/10.1093/bja/76.6.835.
Higgins JPT LT, Deeks JJ (editors). Chapter 6: choosing effect measures and computing estimates of effect. In Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane. 2019, DOI: https://doi.org/10.1002/9781119536604.ch6.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183.
Maddali MM, Mathew J, Fahr J, Zarroug AW. Postoperative nausea and vomiting in diagnostic gynaecological laparoscopic procedures: comparison of the efficacy of the combination of dexamethasone and metoclopramide with that of dexamethasone and ondansetron. J Postgrad Med. 2003;49(4):302–6.
Mathiesen O, Rasmussen ML, Dierking G, Lech K, Hilsted KL, Fomsgaard JS, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand. 2009;53(2):227–35. https://doi.org/10.1111/j.1399-6576.2008.01821.x.
McKenzie R, Riley TJ, Tantisira B, Hamilton DL. Effect of propofol for induction and ondansetron with or without dexamethasone for the prevention of nausea and vomiting after major gynecologic surgery. J Clin Anesth. 1997;9(1):15–20. https://doi.org/10.1016/S0952-8180(96)00215-2.
McKenzie R, Tantisira B, Karambelkar DJ, Riley TJ, Abdelhady H. Comparison of ondansetron with ondansetron plus dexamethasone in the prevention of postoperative nausea and vomiting. Anesth Analg. 1994;79(5):961–4. https://doi.org/10.1213/00000539-199411000-00024.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):e1–37. https://doi.org/10.1016/j.jclinepi.2010.03.004.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. https://doi.org/10.1136/bmj.b2535.
Moore SG. Intravenous dexamethasone as an analgesic: a literature review. AANA Journal. 2018;86(6):488–93.
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. The effect of single low-dose dexamethasone on blood glucose concentrations in the perioperative period: a randomized, placebo-controlled investigation in gynecologic surgical patients. Anesth Analg. 2014;118(6):1204–12. https://doi.org/10.1213/ANE.0b013e3182a53981.
Murphy GS, Szokol JW, Greenberg SB, Avram MJ, Vender JS, Nisman M, et al. Preoperative dexamethasone enhances quality of recovery after laparoscopic cholecystectomy: effect on in-hospital and postdischarge recovery outcomes. Anesthesiology. 2011;114(4):882–90. https://doi.org/10.1097/ALN.0b013e3181ec642e.
Myles PS, Carlisle JB, Scarr B. Evidence for compromised data integrity in studies of liberal peri-operative inspired oxygen. Anaesthesia. 2019;74(5):573–84. https://doi.org/10.1111/anae.14584.
Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, et al. Guidelines for perioperative care in gynecologic/oncology: enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer. 2019;29(4):651–68. https://doi.org/10.1136/ijgc-2019-000356.
Nesek-Adam V, Grizelj-Stojcic E, Rasic Z, Cala Z, Mrsic V, Smiljanic A. Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2007;21(4):607–12. https://doi.org/10.1007/s00464-006-9122-7.
Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31(6):801–16. https://doi.org/10.1016/j.clnu.2012.08.012.
Olajumoke TO, Afolayan JM, Fashanu AO, Adekunle MA. Single dose of dexamethasone for prevention of nausea and vomiting after major gynaecological surgery. Niger J Med. 2013;22(3):207–11.
Ormel G, Romundstad L, Lambert-Jensen P, Stubhaug A. Dexamethasone has additive effect when combined with ondansetron and droperidol for treatment of established PONV. Acta Anaesthesiol Scand. 2011;55(10):1196–205. https://doi.org/10.1111/j.1399-6576.2011.02536.x.
Pajunen P, Vartiainen E, Mannisto S, Jousilahti P, Laatikainen T, Peltonen M. Intra-individual changes in body weight in population-based cohorts during four decades: the Finnish FINRISK study. Eur J Public Health. 2012;22(1):107–12. https://doi.org/10.1093/eurpub/ckq182.
Pan PH, Lee SC, Harris LC. Antiemetic prophylaxis for postdischarge nausea and vomiting and impact on functional quality of living during recovery in patients with high emetic risks: a prospective, randomized, double-blind comparison of two prophylactic antiemetic regimens. Anesth Analg. 2008;107(2):429–38. https://doi.org/10.1213/ane.0b013e318172f992.
Pauls RN, Crisp CC, Oakley SH, Westermann LB, Mazloomdoost D, Kleeman SD, et al. Effects of dexamethasone on quality of recovery following vaginal surgery: a randomized trial. Am J Obstet Gynecol. 2015;213(5):718.e1–7.
Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:CD011770. https://doi.org/10.1002/14651858.CD011770.pub2.
Polderman JA, Farhang-Razi V, Van Dieren S, Kranke P, DeVries JH, Hollmann MW, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018;11:CD011940. https://doi.org/10.1002/14651858.CD011940.pub2.
Rajeeva V BN, Batra Y.K, Dhaliwal L.K. Comparison of ondansetron with ondansetron and dexamethasone in prevention of PONV in diagnostic laparoscopy. Can J Anaesth. 1998;46(1).
Ramesh M KP. Role of dexamethasone in acute postoperative pain, nausea and vomiting in patients undergoing laparoscopic gynocoological surgeries as day case procedures. 2011. p. 725-6.
Rasmussen LS, Yentis SM, Kawamoto M, Shafer SL, Reilly CS, Miller DR, et al. Joint editors-in-chief request for determination regarding papers published by Dr. Yoshitaka Fujii. 2012 [cited 2020 May 26]. Available from: http://journals.lww.com/greenjournal/ Documents/Fujii_Joint_Editorial_Request_ Regarding_Dr_Yoshitaka_Fujii.pdf. .
Regasa T, Aweke Z, Neme D, Hailu S, Jemal B, Mekonen S. Comparison of prophylactic dexamethasone, metoclopramide, and combination of dexamethasone and metoclopramide for prevention of post-operative nausea and vomiting for major gyanecological surgery in Hawassa university compressive specialized hospital, Ethiopia, 2019. Int J Surg Open. 2020;27:18–24. https://doi.org/10.1016/j.ijso.2020.10.004.
Research NIfH. International prospective register of systematic reviews [Internet]. University of York, York, UK.: Centre for Reveiws and Dissemination; 2020 [cited 2020. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=176202.
Rothenberg DM, McCarthy RJ, Peng CC, Normoyle DA. Nausea and vomiting after dexamethasone versus droperidol following outpatient laparoscopy with a propofol-based general anesthetic. Acta Anaesthesiol Scand. 1998;42(6):637–42. https://doi.org/10.1111/j.1399-6576.1998.tb05294.x.
Ryu JH, Chang JE, Kim HR, Hwang JW, Oh AY, Do SH. Ramosetron vs. ramosetron plus dexamethasone for the prevention of postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy: prospective, randomized, and double-blind study. Int J Surg. 2013;11(2):183–7. https://doi.org/10.1016/j.ijsu.2012.12.018.
Samad K, Khan M, Hameedullah KFA, Hamid M, Khan FH. Unplanned prolonged postanaesthesia care unit length of stay and factors affecting it. J Pak Med Assoc. 2006;56(3):108–12.
Sanchez-Ledesma MJ, Lopez-Olaondo L, Pueyo FJ, Carrascosa F, Ortega A. A comparison of three antiemetic combinations for the prevention of postoperative nausea and vomiting. Anesth Analg. 2002;95(6):1590-1595, table of contents.
Sanchez-Rodriguez PE, Fuentes-Orozco C, Gonzalez-Ojeda A. Effect of dexamethasone on postoperative symptoms in patients undergoing elective laparoscopic cholecystectomy: randomized clinical trial. World J Surg. 2010;34(5):895–900. https://doi.org/10.1007/s00268-010-0457-9.
Scott JR. Expression of concern. 2012 [cited 2020 May 26]. Available from: http://journals.lww.com/greenjournal/Documents/ExpressionOfConcern.pdf.
Shrestha BB, Karmacharya M, Gharti BB, Timilsina B, Ghimire P. Effect of Dexamethasone and Pheniramine maleate in patients undergoing elective laparoscopic cholecystectomy. JNMA J Nepal Med Assoc. 2014;52(195):920–4. https://doi.org/10.31729/jnma.2717.
Sistla S, Rajesh R, Sadasivan J, Kundra P, Sistla S. Does single-dose preoperative dexamethasone minimize stress response and improve recovery after laparoscopic cholecystectomy? Surg Laparosc Endosc Percutan Tech. 2009;19(6):506–10. https://doi.org/10.1097/SLE.0b013e3181bd9149.
SS M. Effects of dexamethasone on early postoperative pain, nausea and vomiting and recovery time after ambulatory laparoscopic surgery. J Med Sci. 2007;7(7):1202–5. https://doi.org/10.3923/jms.2007.1202.1205.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Thangaswamy CR, Rewari V, Trikha A, Dehran M, Chandralekha. Dexamethasone before total laparoscopic hysterectomy: a randomized controlled dose-response study. J Anesth. 2010;24(1):24–30. https://doi.org/10.1007/s00540-009-0830-8.
Thomas R, Jones N. Prospective randomized, double-blind comparative study of dexamethasone, ondansetron, and ondansetron plus dexamethasone as prophylactic antiemetic therapy in patients undergoing day-case gynaecological surgery. Br J Anaesth. 2001;87(4):588–92. https://doi.org/10.1093/bja/87.4.588.
Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines N, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) Society Recommendations. World J Surg. 2016;40(9):2065–83. https://doi.org/10.1007/s00268-016-3492-3.
Tolver MA, Strandfelt P, Bryld EB, Rosenberg J, Bisgaard T. Randomized clinical trial of dexamethasone versus placebo in laparoscopic inguinal hernia repair. Br J Surg. 2012;99(10):1374–80. https://doi.org/10.1002/bjs.8876.
Viriyaroj V, Boonsinsukh T, Rookkachart T, Yigsakmongkol N. The effects of single-dose preoperative intravenous dexamethasone on clinical outcome after laparoscopic cholecystectomy. J Med Assoc Thai. 2015;98(Suppl 10):S112–7.
Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200. https://doi.org/10.1093/bja/aes431.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. https://doi.org/10.1186/1471-2288-14-135.
Wang JJ, Ho ST, Liu YH, Lee SC, Liu YC, Liao YC, et al. Dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy. Br J Anaesth. 1999;83(5):772–5. https://doi.org/10.1093/bja/83.5.772.
Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91(1):136–9.
Wang JJ, Ho ST, Uen YH, Lin MT, Chen KT, Huang JC, et al. Small-dose dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy: a comparison of tropisetron with saline. Anesth Analg. 2002;95(1):229-232, table of contents.
World Health Organisation. The ICTRP Search Portal [Internet]. Geneva SWHO. Geneva, Switzerland: World Health Organisation; 2020 [updated 2020 May 29; cited 2020 June 1]. Available from: https://www.who.int/ictrp/search/en/.
Wu JI, Lu SF, Chia YY, Yang LC, Fong WP, Tan PH. Sevoflurane with or without antiemetic prophylaxis of dexamethasone in spontaneously breathing patients undergoing outpatient anorectal surgery. J Clin Anesth. 2009;21(7):469–73. https://doi.org/10.1016/j.jclinane.2008.11.007.
Yuksek MS, Alici HA, Erdem AF, Cesur M. Comparison of prophylactic anti-emetic effects of ondansetron and dexamethasone in women undergoing day-case gynaecological laparoscopic surgery. J Int Med Res. 2003;31(6):481–8. https://doi.org/10.1177/147323000303100603.
Zargar-Shoshtari K, Sammour T, Kahokehr A, Connolly AB, Hill AG. Randomized clinical trial of the effect of glucocorticoids on peritoneal inflammation and postoperative recovery after colectomy. Br J Surg. 2009;96(11):1253–61. https://doi.org/10.1002/bjs.6744.
Acknowledgements
We would like to thank Mrs. Angela Thompson, Librarian, Queen’s University Belfast for her assistance in reviewing the search strategy and Hazel Neale, Healthcare Librarian, Healthcare Library of Northern Ireland at Craigavon Area Hospital, for her assistance in obtaining certain texts.
Funding
Dr. S Bampoe is supported by an award from the NIHR UCLH Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
CM and SB contributed to the conception and design. CM and SJC contributed to the data collection, data analysis and manuscript writing. COD, SB and DW contributed to the manuscript writing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search Strategy
Additional file 2.
List of included items on data extraction form and relevant dropdown list. Dropdown lists were used when possible to reduce the amount of free text.
Additional file 3: Supplementary Table 1.
Table of morphine equivalents used to convert from intravenous or oral opioids to oral morphine
Additional file 4: Supplementary Table 2.
Tabular Risk of Bias Assessment
Additional file 5: Supplementary Figure 1 and Supplementary Figure 2.
Summary risk of bias chart and weighted risk of bias chart.
Additional file 6: Supplementary Figure 3.
Funnel plot for total postoperative opioid requirements.
Additional file 7.
Additional forest plots
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mitchell, C., Cheuk, S.J., O’Donnell, C.M. et al. What is the impact of dexamethasone on postoperative pain in adults undergoing general anaesthesia for elective abdominal surgery: a systematic review and meta-analysis. Perioper Med 11, 13 (2022). https://doi.org/10.1186/s13741-022-00243-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-022-00243-6