Abstract
Spinel-type cobalt oxide (Co3O4) nanoparticles have been easily prepared through a simple thermal decomposition route at low temperature (175°C) using carbonatotetra(ammine)cobalt(III) nitrate complex, [Co(NH3)4CO3]NO3·H2O, as a new precursor. The structure and morphology of as-prepared Co3O4 nanoparticles were characterized by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), UV–vis spectroscopy, Brunauer-Emmett-Teller specific surface area measurement and magnetic measurements, and thermogravimetry/differential thermal analysis. The FT-IR, XRD, and EDS results indicated that the product was highly pure well-crystallized cubic phase of Co3O4. The TEM images showed that the product powder consisted of dispersive quasi-spherical particles with a narrow size distribution ranged from 6 to 16 nm and an average size around 11 nm. The magnetic measurements confirmed that the Co3O4 nanoparticles show a little ferromagnetic behavior which could be attributed to the uncompensated surface spins and/or finite size effects. The ferromagnetic order of the Co3O4 nanoparticles is raised with increasing the decomposition temperature. Using the present method, Co3O4 nanoparticles can be produced without the need of expensive organic solvents and complicated equipments.
Similar content being viewed by others
Background
Nanometer-scale materials with the size of 1 to 100 nm have attracted considerable interest in recent years due to the departure of properties from bulk phases arising from quantum size effects [1]. Spinel-type cobalt oxide (Co3O4) is a technologically important material with applications in lithium ion batteries, heterogeneous catalysts, gas sensing, ceramic pigments, and electrochemical devices [2–11]. This transition metal oxide, when falling in the nanosized regime, is expected to lead to even more attractive applications in the conjunction of their traditional arena and nanotechnology.
In recent years, many efforts have been devoted to the synthesis of Co3O4 nanostructures with different morphologies such as nanoparticles, hollow spheres, nanorods, nanoplates, nanowires, nanotubes, and nanocubes, and nanoporous structures have been prepared [12–22]. Among them, Co3O4 nanoparticles have been prepared by various physical and chemical techniques such as combustion method, microwave irradiation, hydrothermal/solvothermal method, sol–gel process, chemical spray pyrolysis, sonochemical method, polyol method, and so on [23–37]. Most of these methods need some special instruments, harsh conditions, and relatively high processing temperature higher than 350°C. In addition, these methods are either time-consuming or require expensive instruments.
Among various soft chemical methods for preparing nanoscale materials, the thermal decomposition method is widely used due to the process simplicity [38–40]. This technique offers several unique advantages over other methods including easy work-up, low temperature processing, short reaction time, and production of inorganic nanomaterials with narrow size distribution. In recent years, several precursors have been used to synthesize Co3O4 nanoparticles via the thermal decomposition technique [41–45]. However, the most important issue in this technique is to design a precursor which would allow the synthesis of nanomaterials at a low temperature.
In this work, we wish to describe the thermal decomposition of the carbonatotetra(ammine)cobalt(III) nitrate complex, [Co(NH3)4CO3]NO3·H2O, which resulted in the synthesis of Co3O4 nanoparticles at rather low temperature (175°C). The product was identified by various instrumental techniques such as X-ray diffraction (XRD), Fourier transform infrared (FT-IR), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), thermogravimetry/differential thermal analysis (TG/DTA), UV–vis spectroscopy, Brunauer-Emmett-Teller (BET) surface area measurement, and magnetic measurement. This approach provides a one-step, simple, general, and inexpensive method for the preparation of the Co3O4 nanoparticles.
Results and discussion
The FT-IR spectra of the [Co(NH3)4CO3]NO3·H2O complex and its decomposition products at different temperatures are shown in Figure 1. For the complex (Figure 1, curve a), the characteristic stretching bands of NH3, CO3, and NO3 groups are observed at approximately 3,250 to 3,500, 1,600, and 1,350 cm-1, respectively [46]. As shown in Figure 1, curve b, the intensity of these bands decreases when the complex is heated at 150°C. At this temperature, there are two small absorption bands (black square) at about 663.47 and 572.82 cm-1, providing clear evidence for the presence of the crystalline Co3O4[12]. This observation confirms that the formation of Co3O4 nanocrystals begins at approximately 150°C. As can be clearly seen in Figure 1, curve c, with increasing the decomposition temperature to 175°C, only two strong bands assigned to the Co-O stretching of the cubic Co3O4 structure are observed [47]. As shown in Figure 1, curves d,e,f, FT-IR spectra of the samples that were decomposed in the 200°C to 300°C range show only the bands related to the Co3O4 without obvious changes. It is noted that the bands at approximately 3,550 and 1,650 cm-1 in the FT-IR spectrum of some samples should be assigned to the stretching and bending vibrations of the water molecules absorbed by the samples or KBr. Also, there is a tiny band at approximately 2,360 cm-1 on the spectrum of some samples due to the presence of atmospheric CO2.
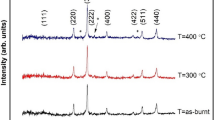
The XRD patterns of the [Co(NH3)4CO3]NO3·H2O complex and its decomposition products at various temperatures are shown in Figure 2. Figure 2a shows the XRD pattern of Co(NH3)4CO3]NO3·H2O precursor. All diffraction peaks appeared in this pattern match very well with those reported in literature for the pure [Co(NH3)4CO3]NO3·H2O (JCPDS 50–1674). As can be seen in Figure 2b, all diffraction peaks related to [Co(NH3)4CO3]NO3·H2O disappeared at 150°C and new broad and weak peaks with 2θ values at 19.50°, 31.37°, 37.02°, 39.10°, 44.97°, 55.84°, 59.58°, 65.46°, and 77.62° appeared. These diffraction peaks can be indexed to the crystalline cubic phase Co3O4 with lattice constants of a = 8.076 Å and a space group of Fd3 m, which are in agreement with the reported values (JCPDS card no. 76–1802). As shown in Figure 2c, the intensity of the characteristic peaks of the Co3O4 phase increases markedly as the temperature increases to 175°C, confirming the complete formation of the Co3O4 phase in good agreement with the FT-IR result. No characteristic peaks of other impurity phases have been detected, indicating that the final product is of high purity. The considerable broadening of the diffraction peaks demonstrates the nanometric character of the particles. The average size of particles prepared was estimated to be about 11.5 nm by the Debye-Scherrer equation [48]: DXRD = 0.9λ/(β cosθ) where DXRD is the average crystalline size, λ is the wavelength of CuKα, β is the full width at half maximum of the diffraction peak, and θ is the Bragg's angle. As we can see in Figure 2d,e,f, no new phase is observed when the decomposition temperature increases to 200°C, 250°C, and then 300°C, but the width of the Co3O4 peaks decreases because of crystallite growth. Further, the increase in the sharpness of peaks with increasing temperature indicates the well crystallization of the product.
The size and shape of the Co3O4 particles prepared by the thermal decomposition of the [Co(NH3)4CO3]NO3·H2O complex at 175°C and also at 200°C were investigated by TEM, as shown in Figure 3a,b. In both cases, the TEM analysis revealed that the samples were formed from extremely fine particles with the sizes of less than 20 nm. It is evident that the particles have uniform size, a homogeneous sphere-like morphology and a narrow size distribution. From the TEM images, it could be concluded that this preparation method is appropriate to obtain the Co3O4 nanoparticles with very small size. The size distribution of the Co3O4 nanoparticles has also been investigated from the particles visualized under TEM analysis. The particle size histograms were determined by counting more than 100 particles in randomly selected regions on the TEM copper grid. The histograms based on TEM analysis are shown in the insets of Figure 3. The inset of Figure 3a shows the particle size distribution of the Co3O4 nanoparticles prepared at 175°C. It can be seen that the particles possess a narrow size distribution in the range of 6 to 16 nm, and the mean particle diameter is approximately 11 nm. Actually, the mean particle size determined by TEM is very close to the average particle size calculated by the Debye-Scherrer formula from the XRD pattern. The particle size distribution was also presented for the Co3O4 nanoparticles prepared at 200°C in the inset of Figure 3b. The size of particles is in the range of 7 to 20 nm with an average particle size of around 13 nm. This value is slightly greater than that of the Co3O4 nanoparticles prepared at 175°C probably due to the crystallite growth at the higher temperature.
The EDS analysis was employed to determine the composition of the product prepared at 175°C. As shown in Figure 4, only cobalt and oxygen elements existed in the product with a Co/O atomic ratio of about 3:3.97 which is consistent with the theoretical value of Co3O4. No other elements can be detected, indicating the high purity of the Co3O4 nanoparticles.
BET surface area measurements were also made on the Co3O4 nanoparticles prepared at 175°C. Figure 5 shows N2 adsorption-desorption which is close to type IV of the IUPAC classification with an evident hysteresis loop in the 0.5 to 1.0 range, suggesting that the sample under study is basically mesoporous. The specific surface area of the sample calculated by the BET method is 73.70 m2 g-1. The relatively high specific surface area of the product is related to the nanometric size of its particles. Assuming that the Co3O4 nanoparticles are almost spherical, as confirmed by TEM, the surface area can be used to estimate the particle size according to the equation DBET = 6000/(ρ × SBET), where DBET is the diameter of a spherical particle (in nm), ρ is the theoretical density of Co3O4 (6.08 g cm-3), and SBET is the specific surface area of the Co3O4 powder in meter squared per gram. The particle size calculated from the surface area data is approximately 13.4 nm, which is in good agreement with the XRD and TEM results.
Optical absorption properties of the Co3O4 nanoparticles prepared at 175°C were investigated at room temperature by UV–vis spectroscopy. Figure 6 shows the absorbance spectrum of the Co3O4 sample with two absorption bands in 250 to 350 and 400 to 580 nm wavelength ranges. The first band can be assigned to the O2- → Co2+ charge transfer process while the second one to the O2- → Co3+ charge transfer [49]. Co3O4 is a p-type semiconductor, and the absorption band gap (Eg) can be determined by the following equation [24]: (Ahν)2 = K (hν - Eg), where hν is the photon energy (eV), A is the absorption coefficient, K is a constant, and Eg is the band gap. The band gap can be estimated by extrapolating the linear region in the plot of (Ahν)2 versus photon energy as shown in the inset of Figure 6. Two absorption peaks give two Eg values for the product, 3.55 and 2.2 eV. As it has been reported in the literatures [50], the Eg values of Co3O4 nanoparticles prepared in this study are greater than those of the bulk Co3O4 (Eg = 3.17 and 1.77 eV, respectively). The increase in the band gaps can be related to the quantum confinement effects and/or small size effects of the Co3O4 nanoparticles [33, 50].
The magnetic measurements for the Co3O4 samples prepared at different temperatures were carried out at room temperature. As shown in Figure 7a, the magnetization curve for the Co3O4 nanoparticles prepared at 175°C exhibits a weak ferromagnetic behavior with a saturation magnetization of 0.137 emu g-1 at the maximum field of 9 kOe applied while the magnetization curves for the Co3O4 samples prepared at 200°C and 250°C in the same figure (Figure 7b,c) display higher ferromagnetic properties with saturation magnetization values of 0.225 and 0.325 emu g-1 at the applied field of 9 kOe, respectively. To confirm that the ferromagnetic behavior originates from the nanoparticles, this measurement was also conducted on a bulk sample. As shown in Figure 7d, the curve exhibits an antiferromagnetic behavior. The ferromagnetic behavior of the nanoparticles can be explained as follows: bulk Co3O4 has a normal spinel structure with antiferromagnetic exchange between ions which occupy the tetrahedral and octahedral sites [51]. It has zero net magnetization owing to the complete compensation of sublattice magnetizations. Hence, the change from an antiferromagnetic state for bulk Co3O4 to a weakly ferromagnetic state for the Co3O4 nanoparticles can be ascribed to the uncompensated surface spins and/or finite size effects [11, 41, 52, 53]. It is well known that the magnetic properties of nanomaterials are strongly dependent on the shape and sizes of their particles, crystallinity, magnetization direction, and so on.
In order to obtain further insight into the nature of the reactions involved in the decomposition pathway of the complex, its thermal behavior was investigated by TG/DTA. Figure 8 shows TG/DTA curves recorded for [Co(NH3)4CO3]NO3·H2O in the 25°C to 600°C temperature range. The TG curve shows that the decomposition of complex proceeds in three stages. The first stage occurred at about 95°C to 110°C and shows 6.68% weight loss which is consistent with the theoretical value of 6.75% caused by the loss of 1 mol of H2O per mole of the complex. The second stage occurs at approximately 135°C to 150°C and shows a 16.75% weight loss, which is consistent with the theoretical value of 16.50% caused by the loss of 1 mol of CO2 per mole of the complex. In the third stage, an extensive weight loss is observed in the range of 160°C to 175°C, which related to the decomposition of the residue complex. Above 175°C, the weight remained constant, confirming the complete decomposition of the complex. The weight loss of all steps that is about 70% is consistent with the theoretical value (70.10%) calculated for the formation of Co3O4 from the complex. The DTA curve for the [Co(NH3)4CO3]NO3·H2O complex as shown in the inset of Figure 8 gave three characteristic peaks in consistent with TG data. The small endothermic peaks at about 100°C can be explained by freeing one H2O molecule. The decomposition of the dehydrated complex was confirmed by one endothermic peak at about 150°C which is immediately followed by a sharp exothermic peak at about 175°C. The endothermic peak at 150°C can be related to the decomposition of unstable bidentate carbonato ligand, and the sharp exothermic peak can be explained by the explosive decomposition of the complex via a redox process taking place between the NH3 ligands as the reductants and NO3- and/or O2 as oxidants. According to the TG/DTG data, the decomposition reactions of the complex can be written as follows:
-
Step 1: [Co(NH3)4CO3]NO3·H2O(s) → [Co(NH3)4CO3]NO3 (s) + H2O (g)
-
Step 2: [Co(NH3)4CO3]NO3 (s) → [Co(NH3)4O]NO3 (s) + CO2 (g)
-
Step 3: [Co(NH3)4O]NO3 (s) + 13/3O2 → 1/3Co3O4 (s) + 6H2O (g) + NO (g) (or NO2) + 2 N2 (g)
Conclusions
In summary, pure and nanosized Co3O4 particles with an average particle size of 11 nm were successfully synthesized by the thermal decomposition of the [Co(NH3)4CO3]NO3·H2O complex as a new precursor at 175°C. Co3O4 nanoparticles are probably formed via the elimination of H2O and CO2 molecules and then explosive redox reaction between the NH3 ligands as the reducing agent and the NO3- ions as the oxidizing agent. By this method, uniform and sphere-like Co3O4 nanoparticles with a narrow size distribution and a weak ferromagnetic behavior can be obtained. The optical absorption band gaps of the Co3O4 nanoparticles were estimated to be approximately 2.20 and 3.55 eV, which are blue shifted in comparison with previously reported values. This method is simple, low cost, safe, and suitable for the industrial production of high-purity Co3O4 nanoparticles for various applications.
Methods
Materials
All materials were of analytical grade and obtained from Merck Company, Merck KGaA, Darmstadt, Germany. Solvents used throughout the reactions were of high purity and used without further purification.
Synthesis of Co3O4 nanoparticles
The precursor complex, [Co(NH3)4CO3]NO3·H2O, was synthesized according to the literature method [54]. To prepare Co3O4 nanoparticles, an appropriate amount of the precursor complex (1 to 2 g) was added to a porcelain crucible and then was placed in an electric furnace. The sample was heated at the rate of 10°C min-1 from room temperature to 150°C in an air atmosphere and then was maintained at this temperature for 1 h. Similar experiments were performed for the samples decomposed in the range of 175°C to 300°C. The decomposition products of the complex at various temperatures were cooled to room temperature and collected for the characterization.
Characterization
The XRD patterns were recorded on a Rigaku D-max C III, X-ray diffractometer (Rigaku Corporation, Shibuya-ku, Japan) using Ni-filtered CuKα radiation (λ = 1.5406 Å) to determine the phases present in the decomposed samples. Infrared spectra were recorded on a Shimadzu system FT-IR 8400S spectrophotometer (Shimadzu Corporation, Kyoto, Japan) using KBr pellets. The optical absorption spectrum was recorded on a Shimadzu 1650PC UV–vis spectrophotometer in the 250 to 700 nm wavelength range at room temperature. The sample for UV–vis studies was well dispersed in distilled water to form a homogeneous suspension by sonication for 25 min. The particle size was determined by a transmission electron microscope (Philips CM10, Philips, Amsterdam, The Netherlands) equipped with a link EDS analyzer. The powders were ultrasonicated in ethanol, and a drop of the suspension was dried on a carbon-coated copper microgrid for the TEM measurements. The specific surface area of the product was measured by the BET method using an N2 adsorption-desorption isotherm carried out at -196°C on a surface area analyzer (Micromeritics ASAP 2010, Micromeritics, Norcross, GA, USA). Before each measurement, the sample was degassed at 150°C for 2 h. Magnetic measurements were carried out at room temperature using a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co. Kashan, Iran). The thermal behavior of the precursor complex was studied using a Netzsch STA 409 PC/PG thermal analyzer (Netzsch, Burlington, MA, USA) at a heating rate of 5°C min-1 in air.
Authors’ information
SF received his BSc degree in Chemistry from Shahid Chamran University in 1991, his MSc degree in Inorganic Chemistry from Tehran University in 1994, and his Ph. D in Inorganic Chemistry from Isfahan University, Iran, in 2000. He is now a professor in inorganic chemistry and the head of the Chemistry Department at Lorestan University, Iran. His research area interests have concentrated on synthesis and characterization of metal and metal oxide nanostructures and their catalytic applications. JS got his BSc in Chemistry in 2009 from the Faculty of Science, Kashan University, Iran. He obtained his MSc in inorganic chemistry in 2012 with the thesis entitled ‘Low-temperature synthesis of Co3O4 nanoparticles from thermal decomposition of the [Co(NH3)4(L)](NO3)n complexes (L = CO3, NO3, H2O; n = 1–3) and their characterization’ from the Faculty of Science, Lorestan University, Iran. PZ obtained her BSc and MSc degrees in Chemistry from the Faculty of Science, Lorestan University, Iran, in 2003 and 2006, respectively. During her MSc course, she has been involved in synthesizing mullite and magnesium aluminate spinel nanopowders by sol–gel process.
Abbreviations
- BET:
-
Brunauer-Emmett-Teller
- EDS:
-
energy-dispersive X-ray spectroscopy
- FT-IR:
-
Fourier transformed infrared spectroscopy
- TEM:
-
transmission electron microscopy
- TG/DTA:
-
thermogravimetry/differential thermal analysis
- XRD:
-
X-ray diffraction.
References
Klabunde KJ, Richards RM: Nanoscale Materials in Chemistry. 2nd edition. Wiley, New York; 2012.
Mate VR, Shirai M, Rode CV: Heterogeneous Co 3 O 4 catalyst for selective oxidation of aqueous veratryl alcohol using molecular oxygen. Catal Commun 2013, 33: 66–69.
Warang T, Patel N, Santini A, Bazzanella N, Kale A, Miotello A: Pulsed laser deposition of Co 3 O 4 nanoparticles assembled coating: role of substrate temperature to tailor disordered to crystalline phase and related photocatalytic activity in degradation of methylene blue. Appl. Catal. A: Gen. 2012, 423–424: 21–27.
Casas-Cabanas M, Binotto G, Larcher D, Lecup A, Giordani V, Tarascon JM: Defect chemistry and catalytic activity of nanosized Co 3 O 4 . Chem Mater 2009, 21: 1939–1947.
Askarinejad A, Bagherzadeh M, Morsali A: Catalytic performance of Mn 3 O 4 and Co 3 O 4 nanocrystals prepared by sonochemical method in epoxidation of styrene and cyclooctene. Appl. Surface Sci. 2010, 256: 6678–6682.
Lou XW, Deng D, Lee JY, Feng J, Archer LA: Self-supported formation of needlelike Co 3 O 4 nanotubes and their application as lithium-ion battery electrodes. Adv Mater 2008, 20: 258–262.
Chou S-L, Wang J-Z, Liu H-K, Dou S-X: Electrochemical deposition of porous Co 3 O 4 nanostructured thin film for lithium-ion battery. J Power Sources 2008, 182: 359–364.
Li YG, Tan B, Wu YY: Mesoporous Co 3 O 4 nanowire arrays for lithium ion batteries with high capacity and rate capacity. Nano Lett 2008, 8: 265–270.
Li W-Y, Xu L-N, Chen J: Co 3 O 4 nanomaterials in lithium-ion batteries and gas sensors. Adv Funct Mater 2005, 15: 851–857.
Sugimoto T, Matijevic E: Colloidal cobalt hydrous oxides, preparation and properties of monodispersed. Co 3 O 4 . J Inorg Nucl Chem 1979, 41: 165–172.
Makhlouf SA: Magnetic properties of Co 3 O 4 nanoparticles. J Magn Magn Mater 2002, 246: 184–190.
Sun L, Li H, Ren L, Hu C: Synthesis of Co 3 O 4 nanostructures using a solvothermal approach. Solid State Sci 2009, 11: 108–112.
Chen Y, Zhang Y, Fu S: Synthesis and characterization of Co 3 O 4 hollow spheres. Mater Lett 2007, 61: 701–705.
Lai T, Lai Y, Lee C, Shu Y, Wang C: Microwave-assisted rapid fabrication of Co 3 O 4 nanorods and application to the degradation of phenol. Catal Today 2008, 131: 105–110.
Wang WW, Zhu YJ: Microwave-assisted synthesis of cobalt oxalate nanorods and their thermal conversion to Co 3 O 4 rods. Mater Res Bull 2005, 40: 1929–1935.
Li L, Chu Y, Liu Y, Song JL, Wang D, Du XW: A facile hydrothermal route to synthesize novel Co 3 O 4 nanoplates. Mater Lett 2008, 62: 1507–1510.
Du J, Chai L, Wang G, Li K, Qian Y: Controlled synthesis of one-dimensional single-crystal Co 3 O 4 nanowires. Aust J Chem 2008, 61: 153–158.
Wang RM, Liu CM, Zhang HZ, Chen CP, Guo L, Xu HB, Yang SH: Porous nanotubes of Co 3 O 4 : synthesis, characterization and magnetic properties. Appl Phys Lett 2004, 85: 2080–2082.
Li Y, Zhao J, Dan Y, Ma D, Zhao Y, Hou S, Lin H, Wang Z: Low temperature aqueous synthesis of highly dispersed Co 3 O 4 nanocubes and their electrocatalytic activity studies. Chem Eng J 2011, 166: 428–434.
Sun H, Ahmad M, Zhu J: Morphology-controlled synthesis of Co 3 O 4 porous nanostructures for the application as lithium-ion battery electrode. Electrochim Acta 2013, 89: 199–205.
Ren M, Yuan S, Su L, Zhou Z: Chrysanthemum-like Co 3 O 4 architectures: hydrothermal synthesis and lithium storage performances. Solid State Sci 2012, 14: 451–455.
Yang LX, Zhu YJ, Li L, Zhang L, Tong H, Wang WW: A facile hydrothermal route to flower-like cobalt hydroxide and oxide. Eur J Inorg Chem 2006, 23: 4787–4792.
Jiu J, Ge Y, Li X, Nie L: Preparation of Co 3 O 4 nanoparticles by a polymer combustion route. Mater Lett 2002, 54: 260–263.
Gu F, Li C, Hu Y, Zhang L: Synthesis and optical characterization of Co 3 O 4 nanocrystals via a facile combustion method. J. Cryst. Growth 2007, 304: 369–373.
Gardey-Merino MC, Palermo M, Belda R, Fernández De Rapp ME, Lascalea GE, Vázquez PG: Combustion synthesis of Co 3 O 4 nanoparticles: fuel ratio effect on the physical properties of the resulting powders. Proced. Mater. Sci 2012, 1: 588–593.
Ai L-H, Jiang J: Rapid synthesis of nanocrystalline Co 3 O 4 by a microwave-assisted combustion method. Powder Tech. 2009, 195: 11–14.
Li L, Ren J: Rapid preparation of spinel Co 3 O 4 nanocrystals in aqueous phase by microwave irradiation. Mater Res Bull 2006, 41: 2286–2290.
Bhatt AS, Bhat DK, Tai C-W, Santosh MS: Microwave-assisted synthesis and magnetic studies of cobalt oxide nanoparticles. Mater Chem Phys 2011, 125: 347–350.
Ma J, Zhang S, Liu W, Zhao Y: Facile preparation of Co 3 O 4 nanocrystals via a solvothermal process directly from common Co 2 O 3 powder. J Alloys Compd 2010, 490: 647–651.
Lester E, Aksomaityte G, Li J, Gomez S, Gonzalez-Gonzalez J, Poliakoff M: Controlled continuous hydrothermal synthesis of cobalt oxide (Co 3 O 4 ) nanoparticles Prog. Cryst. Growth Charact. Mater. 2012, 58: 3–13.
Baydi ME, Poillerat G, Rehspringer JL, Gautier JL, Koenig JF, Chartier P: A sol–gel route for the preparation of Co 3 O 4 catalyst for oxygen electrocatalysis in alkaline medium. J. Solid State Chem. 1994, 109: 281–288.
Kim DY, Ju SH, Koo HY, Hong SK, Kangf YC: Synthesis of nanosized Co 3 O 4 particles by spray pyrolysis. J Alloys Compd 2006, 417: 254–258.
Kumar RV, Diamant Y, Gedanken A: Sonochemical synthesis and characterization of nanometer-size transition metal oxides from metal acetates. Chem Mater 2000, 12: 2301–2305.
Wang X, Chen XY, Gao LS, Zheng HG, Zhang Z, Qian YT: One-dimensional arrays of Co 3 O 4 nanoparticles: synthesis, characterization, and optical and electrochemical properties. J Phys Chem B 2004, 108: 16401–16404.
Fan S, Liu X, Li Y, Yan E, Wang C, Liu J, Zhang Y: Non-aqueous synthesis of crystalline Co 3 O 4 nanoparticles for lithium-ionbatteries. Mater Lett 2013, 91: 291–293.
Jiang J, Li L: Synthesis of sphere-like Co 3 O 4 nanocrystals via a simple polyol route. Mater Lett 2007, 61: 4894–4896.
Zou D, Xu C, Luo H, Wang L, Ying T: Synthesis of Co 3 O 4 nanoparticles via an ionic liquid-assisted methodology at room temperature. Mater Lett 2008, 62: 1976–1978.
Traversa E, Sakamoto M, Sadaoka Y: A chemical route for the preparation of nanosized rare earth Perovskite-type oxides for electroceramic applications. Part Sci Technol 1998, 16: 185–214.
Farhadi S, Rashidi N: Preparation and characterization of pure single-phase BiFeO 3 nanoparticles through thermal decomposition of the heteronuclear Bi[Fe(CN) 6 ] · 5H 2 O complex. Polyhedron 2010, 29: 2959–2965.
Farhadi S, Roostaei-Zaniyani Z: Simple and low-temperature synthesis of NiO nanoparticles through solid-state thermal decomposition of the hexa(ammine)Ni(II) nitrate, [Ni(NH 3 ) 6 ](NO 3 ) 2 , complex. Polyhedron 2011, 30: 1244–1249.
Mohandes F, Davar F, Salavati-Niasari M: Preparation of Co 3 O 4 nanoparticles by nonhydrolytic thermolysis of [Co(Pht)(H 2 O)] n polymers. J Magn Magn Mater 2010, 322: 872–877.
Ren L, Wang P, Han Y, Hu C, Wei B: Synthesis of CoC 2 O 4 · 2H 2 O nanorods and their thermal decomposition to Co 3 O 4 nanoparticles. Mater. Phys. Lett. 2009, 476: 78–83.
Thangavelu K, Parameswari K, Kuppusamy K, Haldorai Y: A simple and facile method to synthesize Co 3 O 4 nanoparticles from metal benzoate dihydrazinate complex as a precursor. Mater Lett 2011, 65: 1482–1484.
Salavati-Niasari M, Khansari A, Davar F: Synthesis and characterization of cobalt oxide nanoparticles by thermal treatment process. Inorg Chim Acta 2009, 362: 4937–4942.
Farhadi S, Pourzare K: Simple and low-temperature preparation of Co 3 O 4 sphere-like nanoparticles via solid-state thermolysis of the [Co(NH 3 ) 6 ](NO 3 ) 3 complex. Mater Res Bull 2012, 47: 1550–1556.
Nakamoto K: Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th edition, Wiley, New York; 2009.
Pejova B, Isahi A, Najdoski M: Fabrication and characterization of nanocrystalline cobalt oxide thin films. Mater Res Bull 2001, 36: 161–170.
Klug HP, Alexander LE: X-ray Diffraction Procedures. 2nd edition. Wiley, New York; 1964.
He T, Chen DR, Jiao XL, Wang YL, Duan YZ: Solubility-controlled synthesis of high-quality Co 3 O 4 nanocrystals. Chem Mater 2005, 17: 4023–4030.
Gulino A, Dapporto P, Rossi P, Fragala I: A novel self-liquid MOCVD precursor for Co 3 O 4 thin films. Chem Mater 2003, 15: 3748–3752.
Ichiyanagi Y, Kimishima Y, Yamada S: Magnetic study on Co 3 O 4 nanoparticles. J Magn Magn Mater 2004, 272–276: e1245-e1246.
Kodama RH, Makhlouf SA, Berkowitz AE: Growth mechanism and magnon excitation in NiO nanowalls. Phys Rev Lett 1997, 79: 1393–1396.
Ozkaya T, Baykal A, Toprak MS, Koseoglu Y, Durmus Z: Reflux synthesis of Co 3 O 4 nanoparticles and its magnetic characterization. J Magn Magn Mater 2009, 321: 2145–2149.
Schlessinger G: Synthesis of carbonatotetra(ammine)cobalt(III) nitrate. Inorg Synth 1960, 6: 173–175.
Acknowledgment
We wish to acknowledge the financial support from Lorestan University Research Council and Iran Nanotechnology Initiative Council (INIC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SF proposed the idea of the study, revised the manuscript critically, and gave final approval for submission. JS was involved in the synthesis and the physicochemical characterization of the Co3O4 nanoparticles and early drafted the manuscript. PZ was involved in the analysis and interpretation of spectral and thermal analysis data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Farhadi, S., Safabakhsh, J. & Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J Nanostruct Chem 3, 69 (2013). https://doi.org/10.1186/2193-8865-3-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-69