Abstract
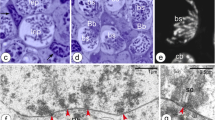
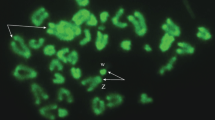
In the females of the viviparous lizard Zootoca vivipara (Lichtenstein, 1823) (family Lacertidae) from Northwest Russia (2n = 35: 32A (acrocentric autosomes) + Z1Z2W sex chromosomes), the ovarian lumen germinal vesicles (oocytes), as well as germinal lamina cells, were examined. Chromosome preparations were obtained using the direct method and the method of total oocyte nuclei spreading developed by Dresser and Moses. Chromosome preparations were stained with Giemsa; for visualization of synaptonemal complexes (SCs), total preparations of oocyte nuclei were stained with silver nitrate and DAPI. It was demonstrated that, during oogenesis in the female, primary follicles enter the early stages of the meiotic prophase I (stages from leptotene to diplotene, lampbrush chromosomes are formed). Here, for the first time, total oocyte spreads were obtained and studied. On the basis of light microscopic analysis of the oocyte SCs and taking into account their length in a female with 2n = 35, the SC karyotype is presented, consisting of 19 SC elements, among which 16 SC autosomal bivalents are distinguished. The remaining three SC elements, according to the authors, can be univalents of Z1Z2W sex chromosomes or one WZ1 bivalent and Z2 and B chromosome univalents. As in the SC karyotype of Z. vivipara males, in a female, specific features in the morphology of SC elements of chromosomes 5 and 6 were observed.



Similar content being viewed by others
REFERENCES
Chmilevskii, D.A., Oogenez ryb v norme i ekstremal’nykh vozdeistviyakh (Fish Oogenesis in Normal and Extreme Conditions), St. Petersburg: S.-Peterb. Gos. Univ., 2017.
Hotta, Y., Ito, H., and Stern, H., Synthesis of DNA during meiosis, Proc. Natl. Acad. Sci. U.S.A., 1966, vol. 66, pp. 1184—1191.
Safronova, L.D. and Kupriyanova, L.A., Metaphase and meiotic chromosomes, synaptonemal complexes (SC) of the lizard Zootoca vivipara, Russ. J. Genet., 2016, vol. 52, no. 11, pp. 1186—1191. https://doi.org/10.1134/S1022795416110120
Surget-Groba, Y., Heulin, B., Guillaume, C.-P., et al., Intraspecific phylogeography of Lacerta vivipara and the evolution of viviparity, Mol. Phylogen. Evol., 2001, vol. 18, pp. 449—459.
Kupriyanova, L., Mayer, W., and Böhme, W., Karyotype diversity of the Eurasian species Zootoca vivipara (Jacquin, 1787) from Central Europe and the evolution of viviparity, in Herpetologia Bonnensis, Vences, M., Kűhler, J., Ziegler, T., and Böhme, W., Eds., Bonn: König Museum, 2006, pp. 67—72.
Cornetti, L., Menegon, M., Giovine, G., et al., Mitochondrial and nuclear survey of Zootoca vivipara across the eastern Italian Alps: evolutionary relationships, historical demography and conservation implications, PLoS One, 2014, vol. 9. e85912
Velekei, B., Lakatos, F., Biro, P., et al., The genetic structure of Zootoca vivipara (Lichtenstein, 1823) populations did not support the existence of a north—south corridor of the VB haplogroup in eastern Hungary, N.-W. J. Zool., 2014, vol. 10, no. 1, pp. 187—189.
Velekei, B., Lakatos, F., Covacin-Marcov, S.-D., et al., New Zootoca vivipara (Lichtenstein, 1823) haplogroup in the Carpatians, N.-W. J. Zool., 2015, vol. 11, no. 2, pp. 336—365.
Kupriyanova, L.A., Safronova, L.D., Tsitrina, A.A., et al., The study of oocytes in the early oogenesis of the viviparous lizard Zootoca vivipara in prophase I meiosis, Russ. J. Dev. Biol., 2019 (in press).
Dresser, M. and Moses, M., Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus gricus): 4. Light and electron microscopy of synapsis and nucleolar development by silver staining, Chromosoma, 1980, vol. 76, pp. 1—22.
Capriglione, T., Olmo, E., Odierna, G., and Kupriyanova, L., Mechanisms of differentiation in the sex chromosomes of some Lacertidae, Amphibia—Reptilia, 1994, vol. 15, pp. 1—8.
Chevalier, M., Dufaure, J., and Lecher, P., Cytogenetic study of several species of Lacerta (Lacertidae, Reptilia) with particular reference to sex chromosomes, Genetica, 1979, vol. 50, no. 1, pp. 11—18.
Belcheva, R., Biserkov, V., Ilieva, H., et al., Karyological studies on Lacerta vivipara (Jacq.) collected in Bulgaria, Cytologia, 1986, vol. 51, no. 3, pp. 567—570.
Kupriyanova, L.A. and Rudi, E., Comparative karyological analysis of viviparous lizard (Lacerta vivipara, Lacertidae, Sauria) populations, Zool. Zh., 1990, vol. 69, no. 6, pp. 93—101.
Orlova, V. and Kozlovskii, A., Heterochromosomes and Nor-localization in the common lizard, Lacerta vivipara Jacquin, from Russia, Russ. J. Herpetol., 1996, vol. 3, pp. 191—195.
Odierna, G., Aprea, G., Capriglione, T., et al., Progressive differentiation of the W sex chromosome between oviparous and viviparous populations of Zootoca vivipara (Reptilia, Lacertidae), Ital. J. Zool., 1998, vol. 65, pp. 295—302.
Kupriyanova, L., Odierna, G., Capriglione, T., et al., Chromosomal changes and form-formation, subspeciation in the wide ranged Eurasian species Zootoca vivipara (evolution, biogeography), in Herpetologia Petropolitana, Ananjeva, N. and Tsinenko, O., Eds., St. Petersburg, 2005, pp. 47—52.
Kupriyanova, L., Kirschey, T., and Böhme, W., Distribution of the common or viviparous lizard, Zootoca vivipara (Lichtenstein, 1823) (Squamata: Lacertidae) in central Europe and re-colonization of the Baltic Sea basin: new karyological evidence, Russ. J. Herpetol., 2017, vol. 24, no. 4, pp. 311—317.
Kupriyanova, L., B-chromosomes in the karyotype of Lacerta parva Boul., Genetica, 1984, vols. 52—53, pp. 223—226.
Kichigin, I., Lisachev, A., Giovannotti, M., et al., First report on B chromosome content in a reptilian species: the case of Anolis carolinensis, Mol. Genet. Genom., 2018. https://doi.org/10.1007/s00438-018-1483-9
Sessions, S., Evolutionary cytogenetics in salamanders, Chromosome Res., 2008, vol. 16, pp. 183—201. https://doi.org/10.1007/810577-007-1205-3
Solovei, I., Gaginskaya, E., Hutchison, H., and Magregor, H., Avian sex chromosomes in the lampbrush chromosomes; the ZW lampbruch bivalents from six species of birds, Chromosome Res., 1993, vol. 1, pp. 153—166.
Kupriyanova, L., Cytogenetic and genetic trends in the evolution of unisexual lizards, Cytogenet. Genome Res., 2010, vol. 127, pp. 273—279.
Petraccioli, A., Guarino, F., Kupriyanova, L., et al., Isolation and characterization of interspersed repeated sequences in the European common lizard, Zootoca vivipara, and their conservation in Squamata, Cytogenet. Genome Res., 2019, vol. 157, no. 2.
ACKNOWLEDGMENTS
We thank O.L. Kolomiets, I.N. Golubovskaya, and V.E. Spangenberg for the attention to the study and valuable advice.
Funding
This study was supported by grant no. AAAA-A19-119020590095-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by N. Maleeva
Rights and permissions
About this article
Cite this article
Kupriyanova, L.A., Safronova, L.D. & Chekunova, A.I. Meiotic Chromosomes, Synaptonemal Complexes in a Female Viviparous Lizard (Zootoca vivipara) in Prophase I of Meiosis. Russ J Genet 55, 774–778 (2019). https://doi.org/10.1134/S1022795419060085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795419060085