Abstract
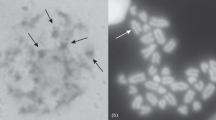
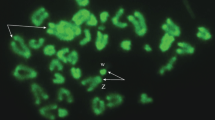
Somatic mitotic and meiotic chromosomes at the pachytene and at the metaphase I of the males of the viviparous lizard, Zootoca vivipara (Lichtenstein, 1823), from northwestern Russia, belonging to the Russian form of Z. v. vivipara, are examined. The spreading of synaptonemal complexes (SC) of their chromosomes are obtained and analyzed for the first time. Eighteen SC are observed, including SC of the Z1Z1 (pairs 5 or 6) and the Z2Z2 (pair 13) sex chromosomes. Characteristics of SC are compared with the number and the shape of bivalents and with those of the karyotype structure. In the studied Russian form of Z. v. vivipara, the length ratios of bivalents correlate with that of mitotic chromosomes (2n = 36); however, some specificity in the morphology of SC of the Z1Z1 sex chromosomes is reported in this article.
Similar content being viewed by others
References
Kupriyanova, L., About possible ways of evolution of lizard karyotype, Tr. Zool. Inst. Akad. Nauk USSR, 1986, vol. 157, pp. 86–101.
Kupriyanova, L., Cytogenetic studies in lacertid lizards, in Cytogenetics of Amphibians and Reptiles, Olmo, E., Ed., Basel: Birkhauser, 1990, pp. 242–245.
Dresser, M. and Moses, M., Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griscus): 4. Light and electron microscopy of synapsis and nucleolar development by silver staining, Chromosoma, 1980, vol. 76, pp. 1–22.
Bogdanov, Yu.F. and Kolomiets, O.L., Karyotyping based on synaptonemal systems and the use of this method in cytogenetics, Genetika (Moscow), 1985, vol. 21, no. 5, pp. 793–802.
Safronova, L.D., Kolomiets, O.L., Bogdanov, Yu.F., et al., The association between synaptonemal complexes of genital and autosomal bivalents in tx/ty male mice as a possible cause of sterility, Genetika (Moscow), 1988, vol. 24, no. 7, pp. 1187–1198.
Wallace, B. and Wallace, H., Synaptonemal complex karyotype of zebrafish, Heredity, 2003, vol. 90, pp. 136–140.
Safronova, L.D. and Krysanov, E.Yu., Synaptonemal complex of two fish species of the genus Nothobranchius (Cyprinodontidae), Russ. J. Genet., 2015, vol. 51, no. 10, pp. 1036–1039.
Reed, K.M., Sites, J.W., and Greenbaum, I.F., Synapsis, recombination, and meiotic segregation in the mesquite lizard, Sceloporus grammicus complex: 1. Pericentric inversion heteromorphism of the F5 cytotype, Cytogenet. Cell Genet., 1992, vol. 61, pp. 40–45. doi 10.1159/000133366
Lutes, A., Neaves, W.B., Baumann, D.P., Wiegräbe, W., and Baumann, P., Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards, Nature, 2010, vol. 464, no. 7286, pp. 283–286.
Chevalier, M., Dufaure, J.P., and Leche, P., Cytogenetic study of several species of Lacerta (Lacertidae,Reptilia) with particular reference to sex chromosomes, Genetica, 1979, vol. 50, pp. 11–18.
Odierna, G., Kupriyanova, L., Capriglione, T., and Olmo, E., Further data on sex chromosomes of Lacertidae and a hypothesis on their evolutionary trend, Amphibia–Reptilia, 1993, vol. 14, pp. 1–11.
Odierna, G., Aprea, G., Capriglione, T., Arribas, O., Kupriyanova, L., and Olmo, E., Progressive differentiation of the W sex-chromosome between oviparous and viviparous populations of Zootoca vivipara (Reptilia,Laceridae), Ital. J. Zool., 1998, vol. 65, pp. 295–302.
Kupriyanova, L., Kuksin, A., and Odierna, G., Karyotype,chromosome structure,reproductive modalities of three Southern Eurasian populations of the common lacertid lizard, Zootoca vivipara (Jacquin, 1787), Acta Herpetol., 2008, no. 3(2), pp. 99–106.
Ashley, T., G-band position effects on meiotic synapsis and crossing over, Genetics, 1988, vol. 118, pp. 307–317.
Srikulnath, K., Matsubara, K., Uno, Y., Nishida, C., Olsson, M., and Matsuda, Y., Identification of the linkage group of the Z sex chromosomes of the sand lizard (Lacerta agilis, Lacertidae) and elucidation of karyotype evolution in lacertid lizards, Chromosoma, 2014, vol. 123, pp. 563–575.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.D. Safronova, L.A. Kupriyanova, 2016, published in Genetika, 2016, Vol. 52, No. 11, pp. 1311–1317.
Rights and permissions
About this article
Cite this article
Safronova, L.D., Kupriyanova, L.A. Metaphase and meiotic chromosomes, synaptonemal complexes (SC) of the lizard Zootoca vivipara . Russ J Genet 52, 1186–1191 (2016). https://doi.org/10.1134/S1022795416110120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795416110120